
DNA: The Genetic Material
- “Transforming factor”: Carries genetic information from generation to generation
- Memory bank: Reserve bank of genetic information
- Single mammalian fetal cell contains few picograms of DNA.
- DNA controls every function of cell through protein synthesis.
- Why DNA: DNA is more stable.
Replication of DNA
- Definition: Replication is a process in which DNA copies itself to produce identical daughter molecules of DNA.
- Model of replication proposed by Watson & Crick (1953)
- High fidelity: Survival of species
- DNA is replicated during S phase of the Cell Cycle
Principles of Replication
- Copying is accurate.
- Replication is semi conservative.
- Super coils and DNA topoisomerases
- Replication fork
- Replication is simultaneous and bidirectional
- DNA synthesis is catalysed by DNA polymerases.
Semi conservative:
- Two complementary strands of parent DNA: simultaneous replication: two daughter molecules.
- New DNA: one from parent strand & one new strand
- Half of original DNA is conserved.
Replication in Prokaryotes
- Most of the information about the replication in prokaryotes is obtained from the studies made on the intestinal bacterium, E.coli.
- The process of replication can be studied conveniently in three stages.
- Initiation
- Elongation
- Termination
Initiation of replication:
- Origin of replication: short sequence of A-T base.
- Multiple sites of origin in eukaryotes. Prokaryotic cells (e.g. E. coli) contain only one origin.
- Specific protein called dna A binds at the site and double stranded DNA gets separated.
- Separation of double stranded DNA forms multiple replication bubbles which is essential for rapid replication process.
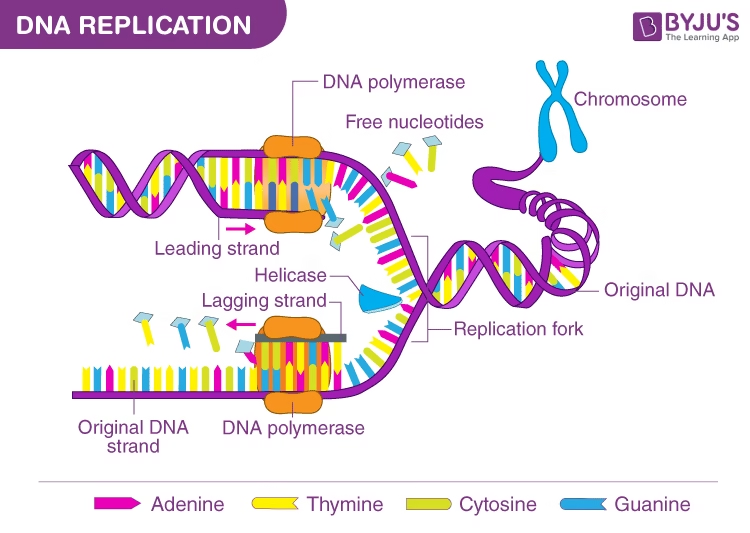 Image: Overview of Replication Process
Image: Overview of Replication Process
- RNA primer (5-50 nucleotides), synthesized on DNA template by a specific RNA polymerase called primase
Elongation:
- In 5’-3’ directions, simultaneously, on both the strands of DNA.
- Its bidirectional from the point of origin.
- Leading strand- continuous. (towards replication fork)
- Lagging strand- discontinuous (away from replication fork)
- Short pieces of DNA (15-250 nucleotides long) produced on lagging strand: Okazaki pieces.
Replication Fork:
- Separation of two strands of parent DNA results in the formation of replication fork.
- Site for active synthesis of DNA.
- Replication fork moves along parent DNA as the daughter DNA molecules are synthesized.
DNA Synthesis:
- DNA Helicases: binds to both the strands and move along the DNA helix to separate the strands. ATP dependent for energy.
- SSB protein (single stranded DNA binding protein): binds to single strand of DNA, keep the two strands separate and provide the template for new DNA synthesis. It also protects the degradation of single-stranded DNA by nucleases.
DNA polymerase III:
- Synthesis of new DNA strand catalysed by DNA polymerase III, occurs in 5’→3’ direction.
- Anti parallel to parent template DNA.
- d ATP, d GTP, d CTP, d TTP- essential prerequisite, added to 3’ end of growing DNA chain.
- Complementary to template DNA strand.
Okazaki pieces
- Small fragments of discontinuously synthesized DNA on lagging strand.
- They are joined by DNA polymerase I & DNA ligase.
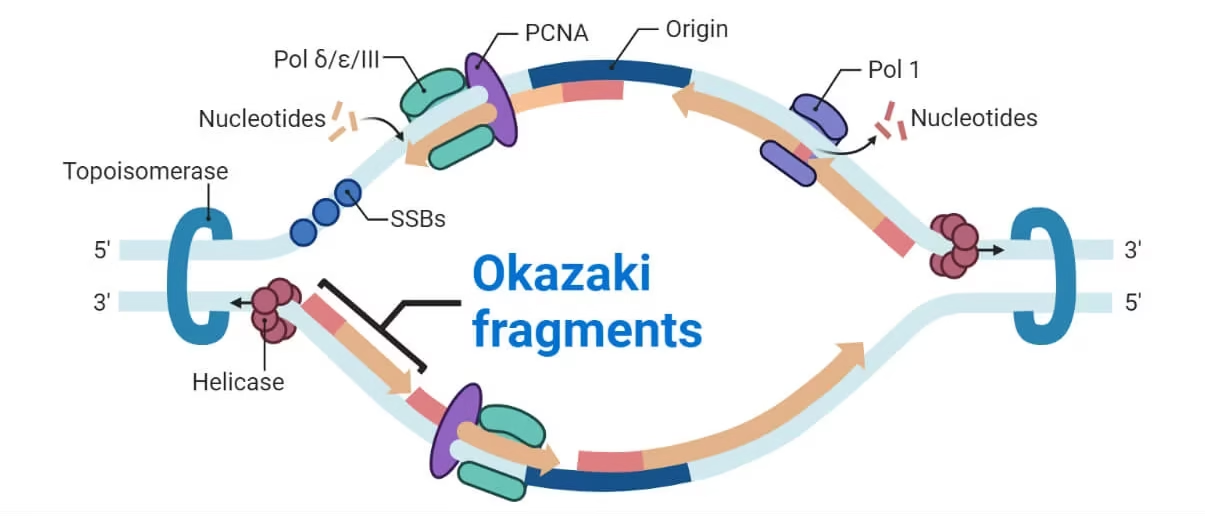
Proof-reading: Fidelity of replication
- DNA polymerase III- checks the incoming nucleotides and allows only the correctly matched bases.
- It also edits the mistakes and removes wrongly placed nucleotide bases.
Replacement of RNA primer by DNA
- DNA polymerase I remove RNA primer and takes its position.
- It catalyses the synthesis of a fragment of DNA that replaces RNA primer.
- Nick sealing – DNA ligase catalyses the formation of a phosphodiester linkage between the DNA synthesized by DNA polymerase III & small fragments of DNA synthesized by DNA polymerase I. It requires energy.
Supercoils and topoisomerases:
- As the double helix of DNA separates from one side and replication proceeds, supercoils are formed at the other side.
Topoisomerases:
- Type I DNA topoisomerases: cuts the single strand- nuclease activity and reseals the strand- ligase activity.
- Type II DNA topoisomerases: cuts both strands and reseal them. Also called as DNA gyrase.
Replication in Eukaryotes:
- There are multiple origins of replication in eukaryotes.
- There are five distinct DNA polymerases identified in eukaryotes. They are numbered by Greek alphabets.
- DNA polymerase alpha synthesises primers for both leading and lagging strands of DNA.
- DNA polymerase beta has similar function as that of DNA polymerase II of prokaryotes –DNA repair.
- DNA polymerase gamma exclusively replicates mitochondrial DNA.
- DNA polymerase delta is analogous to that of DNA polymerase III of prokaryotes. It takes part in the synthesis of both leading and lagging strands with an intrinsic proof-reading activity.
- DNA polymerase epsilon is comparable in its action to the prokaryotic DNA polymerase I. It is involved in the removal of RNA primers of Okazaki fragments on the lagging strand.
Inhibitors of Replication:
- Antibacterial agents:
Ciprofloxacin, Nalidixic acid and Novobiocin – inhibits bacterial DNA gyrase.
- The differences between the DNA replication in bacteria and human cells give scope for developing antibiotics that target bacterial replication but do not affect human cells.
- Anticancer agents:
Etoposide, Adriamycin and Doxorubin inhibit human topoisomerase.
6-mercaptopurine inhibits human DNA polymerase.
5-fluorouracil inhibits Thymydylate synthase.
Central Dogma of Life
In 1958, Francis Crick formulated the much publicized central dogma of life where he proposed the flow of biological information.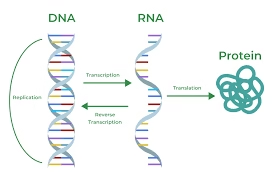
Cells contain three major types of RNA: ribosomal RNA (rRNA) is the chief constituent of ribosomes; messenger RNA (mRNA) carries the information required for protein biosynthesis in its nucleotide sequences; transfer RNA (tRNA) delivers amino acids to the ribosomes for protein biosynthesis of rRNA.
Principles of Transcription
- Transcription is the synthesis of the RNA molecule from DNA.
- Gene: functional unit of the DNA that can be transcribed.
- Roger D. Kornberg won the 2006 Nobel Prize in Chemistry “for his studies of the molecular basis of eukaryotic transcription”
- Product – Primary Transcript (Inactive)
- Post-transcriptional Modifications (splicing, terminal additions, base modifications)
- RNA (Functionally active)
Asymmetric Transcription:
- One of the two strands of DNA serves as a template (sense strand) and produces working copies of RNA molecules.
- The other DNA strand which does not participate in transcription is called as coding (antisense) strand.
 Fig: Coding strand and template strand
Fig: Coding strand and template strand
Selective transcription:
- Unlike replication where the entire sequence of DNA is duplicated, during transcription only certain segments of DNA are expressed.
- The whole DNA- replicated.
- But only small portion of DNA- transcribed.
- For certain regions of DNA – No Transcription
Stages of transcription:
- Initiation
- Elongation
- Termination
RNA polymerase:
- DNA-dependent RNA polymerase: recognition of specific sequences of DNA (σ factor)
- Five polypeptide units- 2α, 1β, 1β’ and one sigma (σ) factor.
- Holoenzyme = Core enzyme (2α,1β,1β’) + σ
Initiation:
- Binding of enzyme RNA polymerase is the prerequisite for the transcription to start.
- RNA polymerase attaches to DNA at a specific region: Promoter region.
- Sigma factor recognizes the promoter region.
- RNA polymerase melts the helical structure.
- Separates 2 strands of DNA; one strand serves as a template.
- RNA polymerase initiates RNA synthesis.
Promoter Regions:
There are two base sequences on the coding strand of DNA which are recognized by sigma factor of RNA polymerase.
- Pribnow box (TATA box): Consists of 6 nucleotide bases (TATAAT) located on the left side about 10 bases away from the starting point of transcription.
- -35 sequence: Base sequence is TTGACA, located about 35 bases away on the left from the site of transcription start.
Elongation:
- RNAP recognizes Promoter region- Sigma factor released- transcription proceeds.
- RNA synthesized from 5’-3’ directions end antiparallel to the DNA template.
- Sequence of nucleotide bases in mRNA is complementary to template DNA; identical to coding strand except U in place of T.
RNA polymerase Difference from DNA polymerase (Replication)
- No primer required.
- No proof-reading activity (do not possess endo- or exo-nuclease activity); No ability to repair the mistakes in RNA Synthesized.
Mistakes in RNA synthesis are less dangerous; since they are not transmitted to daughter cells.
Elongation:
- RNAP Utilizes ribonucleotide Triphosphate (ATP, GTP, CTP, UTP) for RNA formation.
- Supercoils formed due to pushing of polymerase along the template removed by enzymes, gyrase & topoisomerases.
Termination:
- Rho dependant: Rho factor binds to growing RNA- Acts as ATPase- Terminates transcription- Releases RNA.
- Rho independent: Hair pins of newly synthesized RNA are formed due to presence of palindromes, which causes termination.
Transcription process continued till
- RNAP recognizes a termination site on the DNA molecule and releases the new mRNA molecule.
- mRNA leaves the nucleus and travels to the ribosome in the cytoplasm.
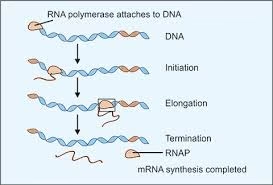 Fig: Transcription process
Fig: Transcription process
Transcription in Eukaryotes
- Eukaryotic transcription is more complex compared to that of prokaryotes. There exist three distinct RNA polymerases transcribing different types of RNAs compared to the single RNAP in prokaryotes.
RNAP I catalyses the formation of large ribosomal RNAs. RNAP II synthesises the primary transcript form of mRNAs. RNAP III takes part in the synthesis of tRNAs and small ribosomal RNAs.
- Two types of sequence elements are identified as eukaryotic promoters
- TATA box
- This sequence is the Goldberg-Hogness box.
- Determines where exactly the transcription has to begin along the template strand of DNA.
- CAAT box
- This region determines how frequently the transcription has to occur and at what speed.
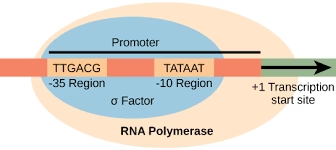 Fig: Promoter region in eukaryotes
Fig: Promoter region in eukaryotes
- Transcription Factors:
- Interact with eukaryotic promoter regions
- Six transcription factors: TFIIA, TFIIB, TFIID, TFIIE, TFIIF, TFIIH.
- Enhancers bind to transcription factors: Activators
The assembly of preinitiation complex (PIC) or basal transcription complex (BTC) [The scale (–30 to +30) shows the extent of BTC].
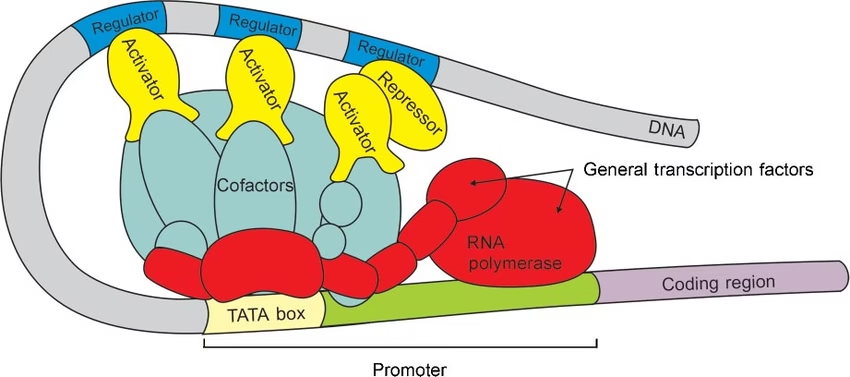 Fig: Eukaryotic basal transcription complex
Fig: Eukaryotic basal transcription complex
hnRNA
- The primary mRNA transcript produced by RNA polymerase II in eukaryotes is often referred to as heterogeneous nuclear RNA.
- It is then processed to produce mRNA needed for protein synthesis.
- Prokaryotic mRNA- Functionally Active
- Eukaryotic mRNA- Post Transcriptional modification
Post transcriptional modifications:
The mRNA in prokaryotes is fully functional as soon as it is synthesised.
On the contrary, all types of eukaryotic RNA produced by transcription as primary transcripts are non-functional as such. They undergo extensive structural alterations to produce the mature functional molecules. The phenomena of RNA processing in eukaryotes are known as post-transcriptional modifications.
- A) Messenger RNA:
- 5’ capping: 7 methylguanosine
- Poly A tail: adenine nucleotide chain at 3’ end.
- Introns and their removal:
- B) Transfer RNA: Trimming, poly A tail
- C) Ribosomal RNA:
Introns and their Removal:
Exons- Possess genetic code & are responsible for protein synthesis.
Introns: Intervening nucleotide sequences that do not code for proteins
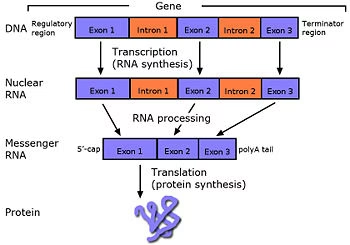 Fig: Intron splicing and removal
Fig: Intron splicing and removal
Inhibitors of Transcription:
- There are a number of drugs and toxins capable of inhibiting the process of transcription at various stages, in bacteria.
- Rifampicin, a drug used in the treatment of tuberculosis and leprosy binds to the RNA polymerase and blocks the bacterial transcription.
- Actinomycin D, an antibiotic, binds to the template strand of DNA and obstructs the movement of RNAP, thus blocking bacterial transcription.
- Alpha-amanitin, a mushroom poison binds to the RNAP II and inhibits the transcription in eukaryotes.
- Heparin, a glycosaminoglycan binds to RNAP and inhibits the transcription
Diseases associated with Transcription:
β-Thalassemia: Faulty splicing
- Due to mutation resulting in nucleotide change at exon-intron junction
- Diminished or lack of synthesis of β–chain of Hb.
mRNA editing:
- Conversion of CAA codon to UAA.
- From same gene, liver synthesizes apoB100 while intestinal cells synthesizes apoB 48.
Reverse Transcription:
- Reverse Transcriptase: RNA dependant DNA Polymerase: RNA→DNA
- Retroviruses: RNA as genetic material
- Oncogenic: Cause cancers in animals
- The mRNA is utilized as a template for the synthesis of double stranded complementary DNA (cDNA)
- This cDNA can be used as a probe to identify the sequence of DNA in genes
Protein Biosynthesis (Translation)
- The genetic information stored in DNA is passed onto RNA through transcription. Genetic information in mRNA (sequence of triplet codon) is translated into a corresponding sequence of amino acids.
- The process of biosynthesis of a protein or a polypeptide in a living cell is known as translation.
- Liver- Protein factory; Erythrocytes: Cannot Synthesize proteins
- Growing & dividing cells: Produce large quantity of proteins.
- 1. Requirement of the components
- 2. Activation of Amino acids
- 3. Protein synthesis proper
- 4. Chaperons and protein folding
- 5. Post-translational modifications
Requirement of the Components:
- Amino acids- monomer of proteins (20)
- Ribosome- Factory or Centres for protein synthesis.
- m RNA- codon (DNA passes genetic information in form of codon to mRNA)
- t RNA- anticodon (Carry AAs & handover to growing peptide chain)
- Energy sources- ATP, GTP
- Protein factors- initiation, elongation and termination factors.
Activation of Amino acids:
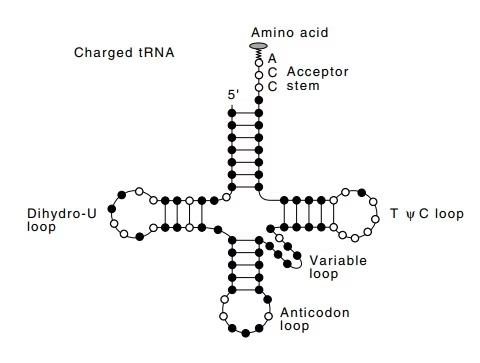 Fig: Activation of amino acids- aminoacyl tRNA synthetase
Fig: Activation of amino acids- aminoacyl tRNA synthetase
- The link between amino acids and nucleic acids is first made by enzymes called aminoacyl tRNA synthetase.
- Enzymes are highly specific for the Amino Acid & corresponding tRNA.
Protein Synthesis (Translation in Eukaryotes):
Eukaryotic translation is complex.
Initiation: Requires eukaryotic initiation factors (eIF)
Elongation: Requires eukaryotic elongation factors (eEF)
Termination: Requires releasing factor (eRF)
- mRNA is read in 5’→ 3’ direction
- Polypeptide synthesis N-terminal end to C-terminal end.
- Translation: directional & collinear with mRNA.
- Prokaryotes: simultaneous transcription & translation
- Eukaryotes: Transcription- Nucleus; Translation- Cytosol.
Initiation:
- Ribosomal Dissociation
- IF-1A & IF-3 bind 40S subunit and separates it from 60S
- Formation of 43S pre-initiation complex
- Complex (IF2-GTP- Met-tRNA) binds
- Formation of 48S initiation complex: mRNA binds
- Formation of 80S initiation complex
- 60S subunit binds; met-tRNA at P site.
- A (aminoacyl t RNA) & P (peptidyl t RNA) sites.
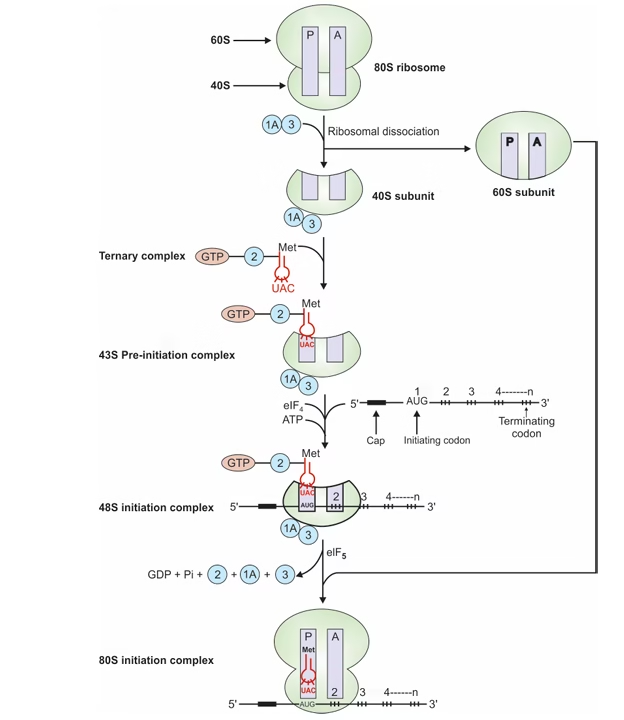 Fig: Initiation of translation in eukaryotes
Fig: Initiation of translation in eukaryotes
Elongation:
- Binding of aminoacyl-tRNA to A-site
Recognition of triplet codon of mRNA by anticodon of corresponding aminoacyl-tRNA.
- Peptide bond formation
Peptidyl transferase splits Met from Met-tRNA at P-site & forms peptide bond between Met & aminoacyl–tRNA at A- site.
- Translocation
GTP translocate peptidyl-tRNA (growing peptide chain) from A to free P-site. New codon now at P-site.
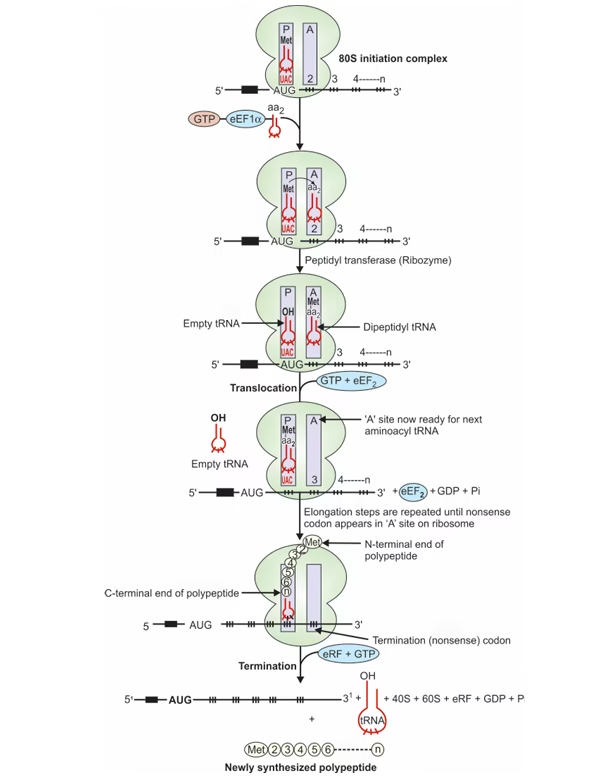 Fig: Elongation and termination in eukaryotic translation
Fig: Elongation and termination in eukaryotic translation
Termination:
- Nonsense codon (UAA,UAG,UGA) at ‘A’ site
- Termination codon occupies A-site, eRF recognizes stop signal.
- eRF-GTP complex, with peptidyl transferase cleaves peptide bond.
Differences between Prokaryotic & Eukaryotic Translation:
| Prokaryotic Translation | Eukaryotic Translation |
| Dietary amino acids not required. | Dietary amino acids required. |
| Ribosome- 50s+30s→70s | Ribosome- 60s+40s→80s |
| 40 t RNA | 50 t RNA |
| m RNA- Polycistronic | m RNA- Monocistronic |
| Initiation factors- IF1, IF2, IF3. | Initiation factors- eIF – 9 in number. |
| m RNA- Shinedalgarno sequence | No such sequence. |
| Methionine is formylated. | Methionine is not formylated. |
| EF- Tu, Ts | eEF |
| RF1, RF2, RF3 | eRF |
Post translational modifications
- Protein Folding
- Chaperones: Heat shock proteins (hsp)
- Facilitate & favour interaction on polypeptide.
- Gives compact & biologically active conformation to proteins.
- Protein misfolding leads to diseases
- Cystic fibrosis: altered protein CFTR
- Prions: aggregates of misfolded proteins (proteinous infectious agents)
- Proteolytic Degradation (trimming)
- In golgi apparatus.
- Zymogen→ active enzymes.
- Preproinsulin→ Insulin
- Intein splicing
- Intervening sequences in certain proteins
- Inteins removed and exteins ligated.
- Covalent Modifications
- A) Phosphorylation:
- Hydroxyl group containing AAs
- Either increase or decrease in activity of proteins
- Protein kinase- Phosphorylation
- Protein phosphatase- Dephosphyrylation
- B) Carboxylation:
- Vitamin K dependent carboxylation of glutamic acid residues of inactive clotting factors
- C) Hydroxylation:
- Proline & Lysine in collagen formation.
- Occurs in endoplasmic reticulum and requires vitamin-C.
- D) Glycosylation:
- Addition of carbohydrate moiety to proteins in synthesis of glycoproteins.
- Serine, threonine, asparagine form glycoprotein on glycosylation.
Inhibitors of Protein synthesis:
- Inhibits initiation: Streptomycin
- Inhibits translocation: Erythromycin, Diphtheria toxin
- Inhibits elongation: Cycloheximide, Chloramphenicol
- Inhibits binding of aminoacyl-tRNA to ribosomal complex: Tetracycline
- Structural resemblance to aminoacyl-tRNA: Puromycin
Genetic code
The three nucleotide (triplet) base sequences in mRNA that code for amino acids in protein are called codons or genetic code.
- Codons composed of four nucleotide bases: A, G, C, U.
- Nucleotide sequence of codon on mRNA: 5’ to 3’ end
- Four bases- 43 = 64 codons.
- 61 codons code for 20 AAs
- AUG (sometimes GUG): Initiation codon.
- Termination Codon- UAA, UAG, UGA
Characteristics of Genetic Code:
- Universality- Same codons for the same amino acid in all species.
- Specificity or Unambiguous- Particular codon always codes for a specific amino acid.
- Degenerate- One amino acid has more than one codon
- Non-overlapping: Commaless & without punctuation.
e.g UGGAUCCAGGAUCCCUUUUAG
- Wobble hypothesis- A single tRNA can recognise more than one codon. This is possible because the third base (3′ base) in the codon sometimes fails to recognise its complementary base in the anticodon (5′ base).
- The pairing of codon and anti codon can wobble (not strict by Watson Crick rule) at third letter e.g. GGU, GGC, GGA are the codes for glycine (synonyms).
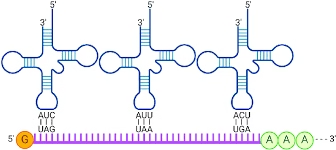 Fig: Codon- anticodon pairing
Fig: Codon- anticodon pairing
Mutations
- Change in the DNA structure of a gene.
- Mutagens- Substances (Chemicals) which cause mutations
Types of mutations:
Mutations can be classified into two major categories.
- Point:
- Replacement of one base pair by another
- It is defined as change in a single nucleotide in DNA. These are sub-classified as
- i) Transitions: In this type, a purine is replaced by another purine or a pyrimidine is substituted by another pyrimidine.
- ii) Transversions: In this case, a purine is replaced by a pyrimidine or vice versa
- Frameshift: Insertion or Deletion Mutations
- Mutations of this type result when one or more base pairs are either inserted in or deleted from the DNA sequences. Hence, they are also called insertion and deletion mutations.
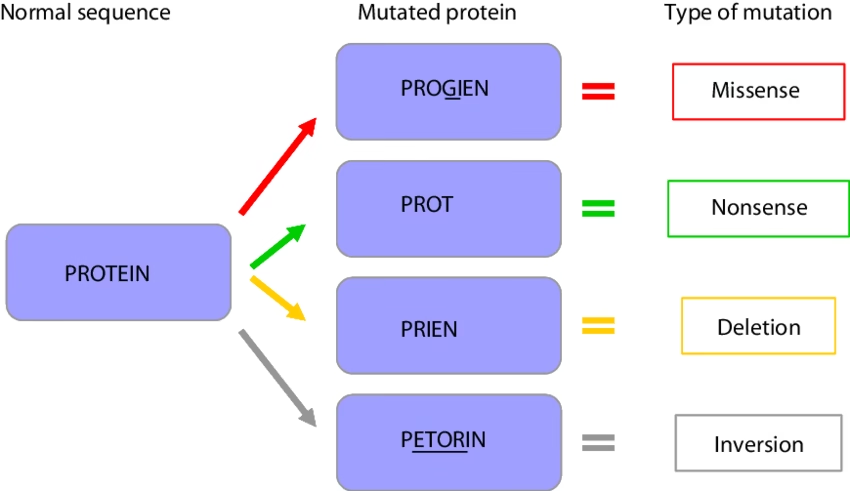
Effects of Point mutations
- Silent mutation
- Missense mutation: The mistaken (or missense) amino acid incorporated into a protein may be acceptable, partially acceptable or unacceptable
- Nonsense mutation
Effects of Frameshift mutations
- Altered reading frame of mRNA.
- Protein synthesized- Altered AAs or premature termination.
Mutations and Cancer
- Mutations are involved in etiopathogenesis of cancer.
DNA damage
The types of damage inflicted on the DNA molecule by various physical and chemical agents (mutagens) are broadly classified into four categories
Types of DNA Damage:
Repair of DNA:
Four major repair systems are involved in rectifying the DNA damage.
- Mismatch repair
- Base excision repair
- Nucleotide excision repair
- Double–strand break repair.
Mechanism of DNA repair:
Disorders of DNA damage:
- Xeroderma Pigmentosum
- Hereditary nonpolyposis colon cancer HNPCC
- Ataxia telangiectasia
- Fanconi’s anaemia
- Cockayne’s syndrome
- Bloom’s syndrome
Regulation of Gene Expression
- Gene: Functional unit of DNA
- Genome: Total genetic information in a cell
- Gene expression:
- Synthesis of proteins under the influence of gene is called gene expression.
- Gene regulation:
- Is essential for growth, development, differentiation & existence of an organism.
- Positive: gene expression increased
- Negative: expression decreased.
- Constitutive/housekeeping genes: required all the time in a cell
- Inducible genes: regulated by molecular signals
- Induction: is the phenomenon of increased synthesis of protein or enzyme in response to certain signal. Such enzymes are said to be inducible and signals are called as inducers. It is also called as derepression.
- Repression: Induction is turning “on” the switch of the gene. Repression is turning “off” the gene expression.
Lac operon:
Operon concept of Gene Regulation:
- Operon is the coordinated unit of genetic expression in bacteria.
- Jacob and Monod put forward the Operon concept in 1961, awarded Nobel prize in 1965.
- Theory is based on the observations on lactose metabolism in E. coli.
- Lactose metabolism is regulated by an induction or derepression process.
Structure of Lac operon
I: Regulatory gene
O: Operator gene
P: Promoter site- RNAP binds
Structural genes code for the enzymes
Z: β-galactosidase
Y: galactoside permease
A: Galactoside acetylase
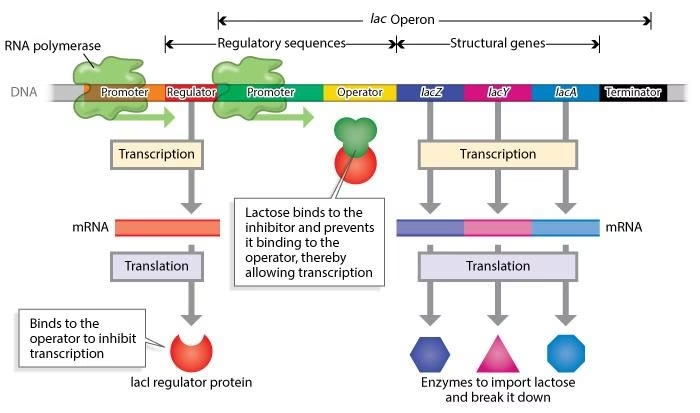 Fig: Diagrammatic representation of the modus operandi of lac operon in E.coli.
Fig: Diagrammatic representation of the modus operandi of lac operon in E.coli.
- Structure of lac operon
- Repression of lac operon
C Derepression of lac operon
(CAP-cAMP = catabolite gene activator protein bound to cAMP; RNAP=RNA polymerase).
Repression of Lac operon:
- Regulatory gene (I): Constitutive: Synthesis of lac repressor (tetrameric protein): Binds to operator gene (0).
- Prevents binding of RNAP to promoter site (P): Blocks transcription of structural genes (Z, Y, A)
- Repressor: Negative regulator of gene expression
Derepression of Lac operon:
- Lactose (inducer) enters E. coli cells: Repressor has high affinity for lactose
- Lactose binds and induces conformational change in Repressor: Inactive repressor.
- Repressor cannot bind O: RNAP binds P: Transcription proceeds: 3 enzymes forms (Z, Y, A).
Lactose act by inactivating the repressor (Derepression of lac operon)
Regulation of Gene Expression in Eukaryotes
- Chromatin structure
- Gene amplification
- Gene rearrangement
- Regulatory elements
- Transcription
- RNA processing
- Alternate mRNA splicing
- Transport of mRNA from nucleus to cytoplasm
- Degradation of mRNA
- Gene regulation at the level of translation
Gene Amplification:
- Amplification of pre-existing genes results in the formation of large number of desired proteins.
- This is necessary during the embryonic developmental stages.
- Methotrexate, the anticancer drug acts by inhibiting the enzyme dihydrofolate reductase. The malignant cells develop drug resistance by amplifying the genes that code for dihydrofolate reductase.
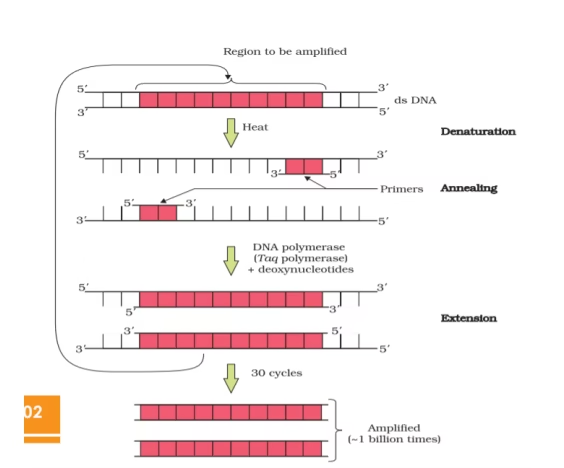 Fig: Diagrammatic representation of amplification of genes
Fig: Diagrammatic representation of amplification of genes
Gene Rearrangement:
- Human beings are capable of synthesizing any number of antibodies against almost any foreign body within a few days of being exposed to it. More than 10 billion distinct antibodies can be formed in response to antigen exposure.
- The specificity of antibody is determined by the amino acid sequences of the variable regions of the both light and heavy chains of immunoglobulins.
- Segmental rearrangement is the key process underlying the synthesis of antibodies.
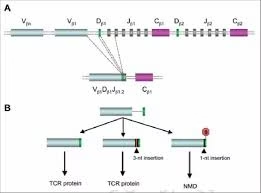 Fig: Gene Rearrangement
Fig: Gene Rearrangement
Gene regulation by Repression:
- Regulatory gene produce Apo-repressor
- Structural gene (enzyme ALA synthase) produces heme (Co-repressor)
- Holo-repressor (Apo+ Co): binds operator: stops transcription.
- When heme (Co-repressor) not available- Repression not effective- Enzyme ALA synthase synthesis (heme) starts.
Genetic engineering
Genetic engineering primarily involves the manipulation of genetic material ( DNA) to achieve the desire goal in pre determined way.
Recombinant DNA technology protocol:
- Isolation of the desired gene
- Isolation of vector
- Formation of chimeric DNA
- Uptake of chimeric DNA (Transfection)
- Selection of cells containing chimeric DNA
- Expression of the gene to produce the desired product
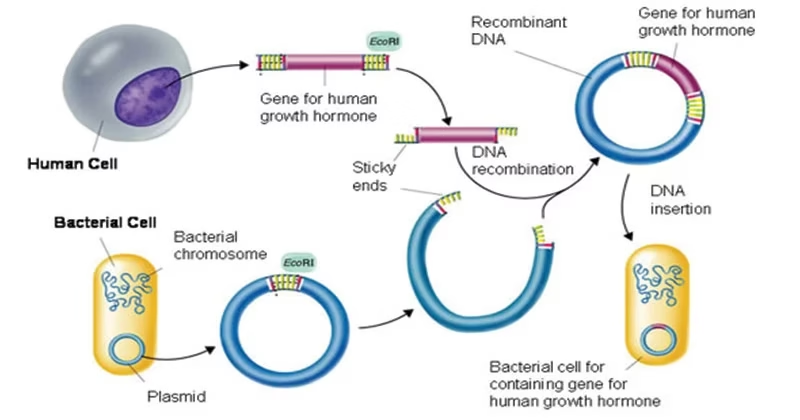 Fig: Basic mechanism of recombinant DNA technology
Fig: Basic mechanism of recombinant DNA technology
Tools of Recombinant DNA Technology:
- Enzymes
- Vectors
- Hosts
- DNA to be cloned
- Enzymes:
- The genetic engineer’s tool kit or molecular tool namely the enzymes are most commonly used in recombinant DNA experiments are
- Restriction endonucleases – DNA cutting Enzyme. Restriction enzymes act as molecular scissors and cut DNA at specific sites called restriction sites
- DNA Ligases- DNA joining Enzyme.
Fig: Commonly used enzymes in recombinant DNA tchnology
- Vectors – The Cloning Vehicles
- Once the desired fragments of DNA are obtained, they are inserted into a suitable vector to produce indefinite number of copies of genes. This is known as gene cloning. They are self-replicating in an appropriate host cell.
- An ideal cloning vector should have the following properties:
- It must have low molecular weight.
- It must have a unique cleavage site for the activity of restriction enzymes at a single point.
- It must be able to replicate truly inside a host cell after its introduction.
- It must contain genes which provide resistance to antibiotics. This helps in the selection of transformed cells from untransformed cells.
The most important vectors are
- Plasmids
- Bacteriophages
- Cosmids
- BAC
- YAC
- HAC
- Host cells – the factor of cloning:
- The hosts are the living system or cell in which the carrier of recombinant DNA molecule or vector can be propagated.
Prokaryotic
- Bacteria: Escherichia coli, Bacillus subtilis, Streptomyces sp
Eukaryotic
- Fungi: Saccharomyces cerevisiae , Aspergillus nidulans
- Animals: Insect cells, Oocytes, Mammalian cells, Whole organisms
- Plants: Protoplast, Intact cell, whole plants
Method of gene Transfer:
- Transformation
- Electroporation
- Conjugation
- Microinjection
- Liposome-mediated gene transfer
Basic techniques in Genetic Engineering:
- Isolation and purification of NA
- Blotting techniques
- Methods of gene transfer
- DNA sequencing
- PCR
- Construction of gene library
- Site directed mutagenesis
Applications of rDNA Technology:
- Insulin
- Hepatitis-B vaccine
- Growth hormone
- Interferons
- Antibiotics
- Recombinant factor VIII
- Tissue plasminogen activator (tPA)
- Monoclonal antiboides
- Diagnosis of HIV infection
- Gene therapy
- Transgenic animals
- In agriculture
Polymerase Chain Reaction (PCR)
PCR is basically a DNA amplification technique. It is an in vitro procedure which generates large quantities of DNA segments. Millions of identical copies of any DNA sequence of interest can be produced by PCR.
Principle and technique of PCR
Step 1 – Denaturation
Step 2 – Renaturation (annealing)
Step 3 – Polymerisation (synthesis)
Applications of PCR:
- Clinical diagnosis:
- PCR is more sensitive in the diagnosis of bacterial and viral infections. For example, in the early phases of tuberculosis, the sputum may contain a very low number of tuberculus bacilli so that normal acid-fast staining may be negative. But PCR can detect even a single bacillus present in the specimen.
- To detect bacterial infection, specific nucleotide sequences of bacilli are amplified by PCR and then detected by Southern blot analysis.
- Reverse PCR is used in diagnosis of viral infections such as hepatitis C and HIV.
- Cancer detection:
- Identification of mutations in oncosupressor genes, such as p53, retinoblastoma gene and so on help in identifying individuals vulnerable to cancer.
- PCR also used to follow up on cancer patients. It is employed to monitor residual abnormal cells present in patients who have been treated.
- Prenatal diagnosis of inherited diseases:
- PCR is employed in the prenatal diagnosis of inherited diseases by using chorionic villus samples of cells from amniocentesis.
- Thus diseases like sickle cell anemia, beta Thalassemia and phenylketonuria can be detected by PCR in these samples.
- Medico-legal cases:
- PCR is so sensitive that it can analyse DNA from a hair follicle or a blood cell. This is particularly helpful in collecting evidence from medicolegal cases (Example: hair follicle from the crime scene is often studied after PCR amplification).
- The obtained pattern of DNA is then compared with the restriction analysis of DNA samples obtained from various suspects; the culprit’s sample will be a perfect match with that of PCR amplified sample.
- The restriction analysis pattern of DNA of one individual will be very specific, hence it is popularly known as DNA fingerprinting; but the pattern will be different from person to person. This is extremely helpful in forensic medicine to identify criminals without error.
- Genetic diseases:
- PCR is widely used in the diagnosis of genetic disorders. The gene segments that contain mutations are amplified to enable diagnosis of inherited diseases such as sickle cell anemia, cystic fibrosis, beta-Thalassemia etc.
- PCR in DNA sequencing:
- PCR is very convenient for use in DNA sequencing. For this single strands of DNA are used.
- Comparative studies of genomes:
- PCR can be used to study the differences in the genomes of two organisms. The products can be separated by electrophoresis for comparative identification.
- Fossil studies:
- DNA can be isolated from fossils and the PCR amplified from it can be used to study evolution by comparing the sequences in the extinct and living organisms.
- Application of PCR in the study of evolution biology (phylogenetics) has great significance, since PCR can amplify even minute quantities of DNA from any source (hair, bone, mummified tissue and so on).
- PCR has revolutionized the evolutionary studies in palaentology and archaeology.
Blotting Techniques
Blotting techniques are commonly used analytical tools for the identification of desired DNA or RNA fragments. Blotting is the process of immobilisation of sample nucleic acids on solid support
Types of Blotting:
The most commonly used blotting techniques are listed below.
- Southern blotting for DNA
- Northern blotting for RNA
- Western blotting for proteins
- Dot blotting (DNA/RNA)