
Micronutrients and Macronutrients:
- Micronutrients are substances required in very small amounts (mg or μg) and that mainly function as co-factors of enzymes ( < 0.005% body weight).
- Vitamins
- Trace minerals (Fe, I, Cu, Mn, Zn, Mo, Se, Mn, F).
- Macronutrients are chemical substances that are required in relatively large amounts (> 0.005% body weight).
Proteins, fats, carbohydrates and minerals (Ca, P, Mg, Na, K, Cl and S).
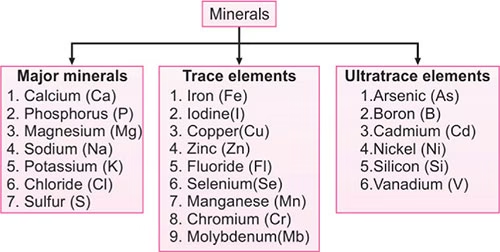 Image: List of Major and trace mineral elements
Image: List of Major and trace mineral elements
Calcium
- Most abundant mineral in the body. (1 to 1.5 kg)
- 99% – bones & teeth, 1% – extracellular fluid.
- Nearly all this calcium occurs in combination with phosphates forming hydroxyapatite crystals that provide inorganic structural component of the skeleton.
Dietary Sources:
- Best: Milk & Milk products
- Good: Beans, Egg yolk, fish, leafy vegetables
RDA:
- Adult men and women: 800 mg/day
- Women during pregnancy, lactation and post-menopause:1.5 gm/day
- Infants: 300-500 mg/day.
Biochemical Functions:
- Development of bones and teeth:
- Along with phosphate- Formation of hydroxyapatite.
- Muscle Contraction: Interacts with Troponin C
- Activates ATPase
- Increase interaction of actin and myosin
- Blood Coagulation: Factor IV dependant on Ca2+.
- Nerve Transmission
- Membrane integrity and permeability
- Activation of Enzymes: Lipase, ATPase
- Calmodulin- Ca binding regulatory protein
- Can bind with 4 calcium ions
- Ca-Calmodulin complex activates certain enzymes
- Intracellular messenger: Second messenger
- Release of Hormones: Insulin, PTH, Calcitonin From endocrine glands
- Secretary Processes: Endocytosis, exocytosis, cell motility
- Contact Inhibition
- Action on heart: Prolongs systole
Factors promoting Calcium absorption:
- Vitamin D: Induces synthesis of Ca binding protein in the intestinal epithelial cells
- Parathyroid hormone: Increased synthesis of Calcitriol.
- Acidity (Low pH)
- Lactose
- Lysine & arginine- AAs
Factors Inhibiting Ca Absorption:
- Phytates and Oxalates
- High content of dietary phosphate.
- Free fatty acids
- Alkaline condition (High pH)
- High content of dietary fiber
- Malabsorption syndrome
Calcium in blood:
- i) Normal blood level
Normal calcium level in plasma 9–11 mg/dl
- ii) Ionised calcium
About 5 mg/dl of calcium is in the ionised form and is metabolically active.
Hormonal regulation (Homeostasis) of calcium metabolism:
There are effective controls to maintain the narrow range (9–11 mg/dl) of blood-calcium.
- Parathyroid hormone [PTH]
- Active vitamin D [1, 25(OH)2 D] or Calcitriol
Primary hormone regulating bone and Mineral metabolism
- Calcitonin
- Calcitriol- Increases blood calcium level
- PTH- Increases blood calcium level
- Calcitonin- Decreases blood calcium level
- Calcitonin, Calcitriol and PTH act together to achieve calcium homeostasis
Disorders of calcium metabolism:
Hypercalcemia:
- Serum calcium is greater than 11 mg/dl.
- Increased serum Ca level is associated with hyperparathyroidism.
- Decrease in serum phosphate (increased urinary losses) & increase in alkaline phosphatase.
- Elevated urinary excretion of Ca & P- formation of urinary calculi
Hypocalcemia:
- Serum calcium is less than 9 mg/dl
- More serious and life threatening
- Hypocalcemia is mostly due to Hypoparathyroidism. (After accidental removal of parathyroid glands )
- Tetany: Serum calcium below 7 mg/dl
- Neuromuscular irritability, spasms, convulsions, Chvotek’s sign, Trousseau’s sign
Rickets:
- Incomplete mineralization resulting in soft and pliable bones and delay in teeth formation.
- Weight bearing bones bent to form bow-legs.
- Cause: Low levels of Vitamin D in body or dietary deficiency of Ca or P or both.
- Increase in activity of alkaline phosphatase
Phosphorus
- 80%- bones & teeth
- 10%- muscles
Sources –Cheese, Milk, Nuts, Eggs, Cereals
Daily requirement:
- Ratio of Ca:P 1:1 Recommended
- Adults- 500mg
- Pregnant women-1gm
- Children- 400-600mg
Biochemical Functions:
- Important constituent of bones and teeth
- Component of high energy phosphates-ATP,CTP,GTP and creatinine phosphate.
- Synthesis of nucleotide coenzymes such as NAD+, NADP+ and PLP.
- DNA & RNA have phosphodiester linkages (backbone of structure)
- Certain proteins and enzymes activated by phosphorylation
- Phospholipids, phosphoproteins-contain phosphate.
- Phosphate buffer system
Disease States:
- Hyperphosphatemia: Hypoparathyroidism
- Hypophosphatemia:
Iron
- Total body iron content- 3-5gm
- About 75% of this occurs in the RBC as a constituent of hemoglobin.
Heme- predominant iron containing substance
- Constituent of several proteins/enzymes
- hemoglobin, Myoglobin, cytochrome, xanthine oxidase, catalase, tryptophan pyrrolase, peroxidase
Dietary requirements
- Adult male – 10 mg/day
- Menstruating woman – 20 mg/day
- Pregnant and lactating women – 40 mg/day
Dietary Sources:
- Rich :Organ meats (liver, heart, kidney), jaggery
- Good: Leafy vegetables, pulses, cereals, fish, apples, dates
- Poor-:Milk, wheat, polished rice
Biochemical functions:
- Iron exerts its functions through the compounds in which it is present.
- The major role of iron in humans is to carry O2 to tissues and CO2 to lungs as part of heme protein that in turn is part of hemoglobin. Oxygen is also bound by another iron-containing heme protein in muscle, myoglobin.
- Peroxidase-required for phagocytosis and killing of bacteria by neutrophils
- Component of the enzyme catalase in RBC which exerts an antioxidant role.
- Effective immunocompetance of body
- Vital role in mitochondrial electron transport (cellular respiration) as a component of cytochrome and iron–sulphur proteins.
Absorption, transport, storage:
- absorbed mainly in stomach and duodenum
- 10% of dietary iron is absorbed
- In Foods-iron in ferric form-bound to proteins/organic acids
- Reducing substances-vitamin C & cysteine convert ferric to ferrous[soluble form]
Factors affecting Fe absorption:
- Acidity, ascorbic acid, alcohol, fructose and cysteine promote iron absorption.
- In iron deficiency anemia, Fe absorption is increased and is almost ten times that of normal absorption.
- Amino acids and small peptides favour iron uptake by mucosal cells.
- Phytates (in cereals), oxalates (in leafy vegetables) and tannins (in tea) inhibit Fe absorption by forming insoluble iron salts.
Fig: Iron absorption, transport, storage
Iron-one way substance:
- Homeostasis is maintained by regulation at the level of absorption & not by excretion
- No other nutrient is regulated in this manner
- Mucosal block theory
- Depleted iron stores>>absorption of iron enhanced
- Adequate iron stores>>absorption decreased
- Iron losses from body are minimal [<1mg/day] through bile, sweat, hair loss
- Iron not excreted into urine
- Iron differs from vitamins/ organic/ inorganic substances which are inactivated or excreted during metabolic function
Disease states:
Iron deficiency anaemia:
- Most prevalent nutritional disorder worldwide
- Microcytic hypochromic anaemia
Causes:
- Nutritional deficiency of iron
- Lack of absorption
- Hookworm infection
- Repeated pregnancies
- Chronic blood loss
- Lead poisoning-
Hemosiderosis:
- Excessive iron in the body
- Subjects receiving repeated transfusions
Hemochromatosis:
- Bronze diabetes
- Iron directly deposited in the tissues (Liver, spleen, pancreas, skin)
Sodium
- Chief cation of extracellular fluid
- 50%-bones
- 40%-extracellular fluid
- 10%-soft tissues
Dietary sources: Common salt (NaCl) used in cooking medium is the major source of sodium. Whole grains, nuts, eggs, leafy vegetables, milk and bread are good sources of Na+.
Dietary requirements: RDA of sodium is about 5–10 g/day.
Sodium in ECF: The normal concentration of sodium in plasma is 135–145 mEq/L.
Biochemical functions:
- Sodium regulates the body’s acid–base balance along with chloride and bicarbonate. It is involved in forming a bicarbonate buffer system (NaHCO3–H2CO3) and a phosphate buffer system (NaH2PO4–Na2HPO4). These buffer systems play an important role in the acid–base balance.
- Sodium plays a role in maintaining osmotic pressure and fluid balance.
- Sodium is important in muscle excitability and is necessary for initiating and maintaining the heartbeat.
- Necessary for normal muscle irritability and cell permeability.
- Intestinal absorption of glucose, galactose, amino acids
Diseases states:
Hyponatremia
When the plasma sodium level falls below normal, the condition is referred to as hyponatremia.
The causes include
- Vomiting
- Diarrhoea
- Burns
- Addison’s disease (adrenocortical insufficiency)
- Renal tubular acidosis (tubular reabsorption of sodium is defective)
- Severe sweating
Symptoms- reduced BP, circulatory failure
Hypernatremia
This condition is marked by an elevation in the plasma sodium level.
Causes of hypernatremia include
- Cushing’s syndrome (adrenocortical hyperactivity)
- Prolonged cortisone therapy
- Pregnancy (steroid hormones cause sodium retention in the body to some extent)
- Dehydration (when water is lost, the blood volume is decreased with apparent increase in the concentration of sodium- diabetes insipidus)
Potassium
Potassium is the major intracellular cation.
Dietary sources: Banana, orange, pineapple, potato, beans, chicken and organ meat (liver) are good sources and coconut water has very high content of potassium.
RDA: About 3–4 g/day.
Potassium in the ECF and ICF: The plasma concentration of potassium is 3.5–5.0 mEq/L.
- Absorption- Na-K pump
- Excreted in urine
Biochemical functions:
- Potassium maintains intracellular osmotic pressure. Movement of water across the biological membranes is dependent on the osmotic pressure differences between ICF and ECF. In a healthy state, the osmotic pressure of ECF (mainly due to Na+ ions) is equal to the osmotic pressure of ICF (contributed by K+ ions). As such, there is no net passage of water molecules in or out of the cells maintaining osmotic equilibrium
- It plays a role in the regulation of acid–base and water balances in the cells.
- The enzyme, pyruvate kinase (glycolysis) is dependent on K+ for its optimal activity.
- Potassium is required for the transmission of nerve impulses.
- For biosynthesis of proteins in ribosomes.
Disease states:
Hypokalemia:
- Hypokalemia is manifested as muscular weakness, impaired myocardial contractility leading to cardiac arrhythmias and even cardiac arrest.
- Hypokalemia is observed in hyperactivity of the adrenal cortex (Cushing’s syndrome).
Hyperkalemia
- Hyperkalemia, leads to ventricular arrhythmia, ventricular fibrillation, bradycardia and may lead to cardiac arrest.
- Decreased potassium excretion can occur in mineralocorticoid deficiency (Addison’s disease) and by potassium sparing diuretics.
Copper
Copper mostly distributed in the liver, brain, kidneys, heart, bone, muscle and hair.
Dietary sources and requirements
- Organ meats, cereals, fruits, green leafy vegetables, shellfish, poultry are sources of dietary copper.
RDA:
- Adults : 2–3 mg/day
- Infants and children: 0.5 –2 mg/day
Biochemical functions:
- As the metal cofactor for many enzymes.
Image: Copper containing enzymes
- As a component of cytochrome oxidase (complex IV of ETC), it plays a role in cellular respiration.
- As a constituent of superoxide dismutase and catalase, it is involved in antioxidant function.
- As a cofactor of ALA synthase (heme synthesis), it is required for the formation of hemoglobin, myoglobin and cytochromes.
- The hydroxylation of lysine and proline (required for collagen cross-linking) requires copper as an essential cofactor.
Disease States:
Menke’s kinky hair syndrome
- It is an X-linked condition (affecting only male infants).
- It is said to be due to mutations in the gene for a copper -binding ATPase.
- As a result, copper cannot be transported in the blood.
Wilson’s disease
- It is a genetic disease (autosomal recessive) which occurs due to mutations similar to that of Menke’s disease but in a different gene (a gene encoding a copper-binding ATPase expressed only in liver cells).
- Hence, the condition is also known as hepatolenticular degeneration.
- Ceruloplasmin levels in blood are decreased.
- Kayser–Fleischer ring: pigments that occur in Wilson’s disease are observed in cornea of the eye.
- Patients with Wilson’s disease are treated with lifelong administration of penicillamine and British antilewisite (BAL) which chelate copper, and are subsequently excreted in urine
Iodine
- Occurs in the thyroid gland
Dietary sources
- Seafoods and plant foods grown near the sea are the best sources. Iodine content in the soil and water is very low in high altitudes. There are many areas around the world, endemic for iodine deficiency. In all other areas, drinking water, vegetables and fruits contain moderate amounts of iodine. Nowadays, iodised salt is used for cooking in most households.
Dietary requirements of iodine:
- Adults : 100–150 μg/day
- Pregnant and lactating women: 200 μg/day
- Protein-bound iodine (PBI)
Biochemical functions:
- Iodine is used for the synthesis of thyroid hormones namely, thyroxine (T4) and triiodothyronine (T3).
Disease states:
- Goitre (enlargement of thyroid gland). Hypothyroidism and myxedema occur in iodine deficiency. In children, cretinism may occur.
Zinc
- Total content in body- 2g
- Prostate gland rich in zinc
- Dietary requirement- 10-15mg/day
- Sources- meat, fish, eggs, milk, beans, nuts
Biochemical Functions:
- Essential component of several enzymes
- carbonic anhydrase,
- alcohol dehydrogenase,
- alkaline phosphatase,
- carboxypeptidase,
- superoxide dismutase
- Lactate dehydrogenase
- DNA and RNA polymerase
- It plays a crucial role in the synthesis and stabilisation of DNA, RNA and proteins. It is an integral component of DNA and RNA polymerases. It forms zinc fingers.
- Antioxidant-due to Superoxide dismutase
- Storage & secretion of insulin from beta cells of pancreas require Zn
- Zn promotes synthesis of retinol binding protein
- Necessary to maintain normal levels of vit A
- Required for wound healing, enhances cell growth
- As a component of carbonic anhydrase, zinc plays an important role in blood-gas transport and renal regulation of pH.
- Gusten, Zn containing protein of saliva important for taste sensation
- Gusten is necessary for normal development of taste buds
- Essential for proper reproduction
- It is required for embryonic development and fetal growth.
Inherited metabolic disease of zinc deficiency: Acrodermatitis enteropathica
Fluorine
- Fluoride found in bones & teeth
- Dietary requirement <2ppm
- Source– drinking water
Biochemical Functions:
- Prevents development of dental caries
- Forms a protective layer of acid resistant fluoroapatite with hydroxyapatite of enamel
- Fluoride inhibits bacterial enzymes, reduces production of acids
- Fluoride increases the resistance of enamel to acid attacks.
- Fluoride is essential for the normal development of bony structure.
- Sodium fluoride inhibits enolase [of glycolysis]
- Fluoroacetate inhibits aconitase [TCA cycle]
Disease States:
- Dental caries: drinking water <0.5ppm of fluoride associated with dental caries
- Fluorosis: drinking water >2ppm of fluoride
- In children causes-mottling of enamel, discoloration of teeth
- Dental fluorosis– teeth become weak, rough, brown or yellow patches
- Skeletal fluorosis: >20ppm fluoride is toxic
- Hypercalcification , increased density of bones of limbs, pelvis, spine
- Ligaments of spine get calcified
- Advanced stage- individuals get crippled, stiff joints, Genu valgum
Selenium
Dietary sources and requirements:
Seafood, muscle meat and all kinds of cereals are rich sources of selenium.
Dietary requirement is about 50–100 µg/day.
Biochemical Functions
- Along with vitamin E prevents development of hepatic necrosis and muscular dystrophy
- Maintain structural integrity of biological membrane
- Selenium as selenocystiene [21st AA]
- Essential component of glutathione peroxidase
- Selenium is also a constituent of thioredoxin reductase
- Prevents lipid peroxidation
- Binds with heavy metals [Hg,Cd]-protects body from their toxic effects
- Selenium containing enzyme-5’deiodinase converts T4 to T3
- Selenium is involved in the removal of iodine from thyroid hormones.
Deficiency- Keshan’s disease- endemic cardiomyopathy
Toxicity- Selenosis