Introduction
- Proteins are the prime molecules of life occurring in every cell and every part of the cell.
- Proteins are the building material for the body structure.
- They account for about half of the organic material of the human body. (17% of body weight).
- Proteins are composed of carbon, oxygen, hydrogen, nitrogen and small amounts of other elements, notably sulphur.
- Proteins are important sources of nitrogen. (16% of protein is nitrogen).
Amino acids
- Structural units (monomers) of proteins.
- Amino acid is made up of two functional groups—amino (–NH2), carboxyl (–COOH).
- They also contain a hydrogen atom and a side chain (R) linked to the carbon atom.
- Amino acids differ from each other in their side chains.
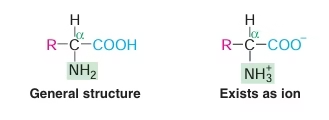 Fig: General structure of Amino Acids
Fig: General structure of Amino Acids
Classification of Amino Acids
Nutritional classification of Amino Acids
Essential amino acids
- Amino Acids cannot be synthesized by the body and therefore need to be supplied through the diet.
- They are required for proper growth and maintenance of the individual.
-
- Valine
- Isoleucine
- Leucine
- Lysine
- Methionine
- Phenylalanine
- Threonine
- Tryptophan
Semi essential amino acids
- Synthesized by adults but not by growing children. So, essential for growing children.
-
- Histidine
- Arginine
Nonessential amino acids
- They are not required in diet as they are synthesized by the body.
-
- Glycine
- Alanine
- Serine
- Cysteine
- Aspartate
- Aspargine
- Glutamate
- Glutamine
- Tyrosine
- Proline
Amino acid classification: Based on Structure and Chemical reaction:
Amino acids with aliphatic side chains
-
- Glycine
- Alanine
- Valine
- Leucine
- Isoleucine
Amino acids containing hydroxyl (–OH) groups
-
- Serine
- Threonine
Sulphur containing amino acids
-
- Cysteine
- Methionine
Acidic amino acids and their amides
-
- Aspartic acid
- Asparagine
- Glutamic acid
- Glutamine
Basic amino acids
-
- Lysine
- Arginine
- Histidine
Aromatic amino acids
-
- Phenylalanine
- Tyrosine
- Tryptophan
Imino acid
-
- Proline
Amino acid Classification: Based on Metabolic Fate:
Ketogenic amino acids
-
- Leucine is the only exclusive ketogenic amino acid.
Ketogenic and glucogenic amino acids
-
- Isoleucine, lysine, phenylalanine, tyrosine and tryptophan.
Glucogenic amino acids
-
- The remaining 14 amino acids are used for the synthesis of glucose or glycogen.
Amino acid Classification: Based on Solubility:
- Polar (hydrophilic) amino acids
glycine, serine, threonine, cysteine, asparagine, glutamine and tyrosine. - Non-polar (hydrophobic) amino acids
Phenyl alanine, tryptophan and proline
Properties of amino acids
- Amino acid can act as acids & bases
- Amino acids may have positive, negative or zero net charge
- At isoelectric pH (pI) Amino acid bears no net charge
- Amino acids have characteristic titration curve. It predicts electric charge of amino acid.
- pka values express strength of amino acid which vary with environment
Chemical Properties
REACTIONS DUE TO –COOH GROUP
-
- Salts(-COONa) with bases,esters(-COOR’) with alcohols
- Decarboxylation
- Reaction with ammonia
REACTIONS DUE TO –NH2 GROUP
-
- Reaction with ninhydrin
- Colour reactions of amino acids
- Transamination
- Oxidative deamination
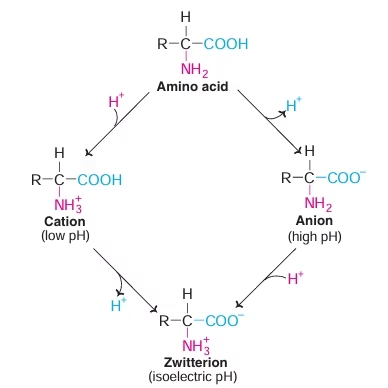 Fig: Isoelectric pH
Fig: Isoelectric pH
Biologically Important Peptides
- Peptides displaying wide spectrum of biological functions
- Constituent amino acid number less than 10
- Also called as Biologically Active Peptides
- Glutathione
-
- Tripeptide
- γ-glutamyl-cysteinyl-glycine
- Exists in reduced and oxidized states.
- 2G-SH→→→→→ G-S-S-G
- Reduced ←←←← Oxidized
-
- Coenzyme for certain enzymes e.g. PGE2 synthetase
- Prevents oxidation of sulfhydryl Essential for proteins and enzymes function.
- Maintain RBC membrane structure and integrity.
- Protects Hemoglobin from getting oxidized.
- Scavenging of peroxides and free radicals.
- Transport of amino acids in intestine and kidney (Meister cycle)
- In Detoxification- Phase II conjugation reactions: Glutathione is Conjugating agent
- Thyrotropin releasing hormone:
-
- Tripeptide
- Secreted by hypothalamus
- Stimulates pituitary to release thyrotropic hormone
- Oxytocin
-
- Nona-peptide
- Secreted by posterior pituitary
- Causes contraction of uterus
- Vasopressin
-
- Nonapeptide
- Produced by posterior pituitary
- Stimulates kidney to retain water
- Increases blood pressure
- Angiotensin I and II
-
- Decapeptide and Octapeptide
- Angiotensin II stimulates release of aldosterone from adrenal gland
- Hypertensive effect
- Methionine enkephalin
-
- Pentapeptide found in brain
- Opiate like function
- Inhibits sense of pain
- Bradykinin and kallidin
-
- 9 and 10 amino acids respectively
- Powerful vasodilators
- Produced from plasma proteins by snake venom enzymes
8. Peptide antibiotics
-
- Gramicidin, bacitracin, tyrocidin, actinomycin
9. Aspartame
-
- Dipeptide –aspartyl phenylalanine methyl ester
- Artificial sweetner
10. Gastrointestinal hormones
-
- Gastrin, secretin
Proteins
- Greek word ‘proteios’=holding the first place.
- Most abundant organic molecules of the living system.
- Occur in every part of the cell and constitutes 50% of the cellular dry weight.
- Fundamental basis of structure and function of life.
- Proteins are polymers of amino acids.
Functions/ Biomedical Importance:
Structural (Static):
- Responsible for structure and strength of the body. (Collagen, elastin in bone)
Dynamic:
- Enzymes, hormones, blood clotting factors, immunoglobulin and membrane receptors.
- Working horses of the cell.
Elemental composition of Proteins:
- Carbon : 50-55 %
- Hydrogen: 6-7 %
- Oxygen : 19-24%
- Nitrogen : 13-19%
- Sulfur : 0-4 %
Protein Classification
- Based on Chemical Nature
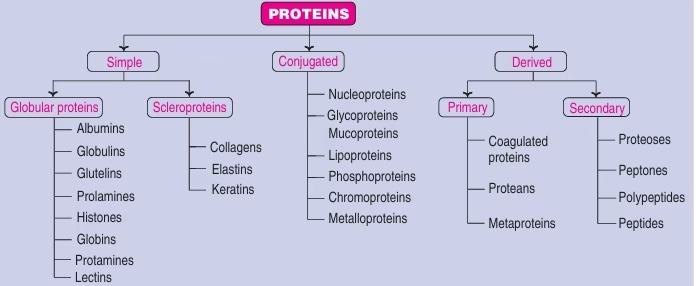 Fig: Protein classification based on chemical nature
Fig: Protein classification based on chemical nature
- Functional Classification:
- Structural: Keratin (hair, nails), Collagen (Bone)
- Enzymes or catalytic: Pepsin
- Transport: Hemoglobin, Albumin
- Hormonal: Insulin, Growth hormone
- Contractile: Actin, Myosin
- Storage: Ovalabumin.
- Genetic: Nucleoproteins
- Defence: Immunoglobulins
- Receptor for Hormones.
Structural Organization of Proteins
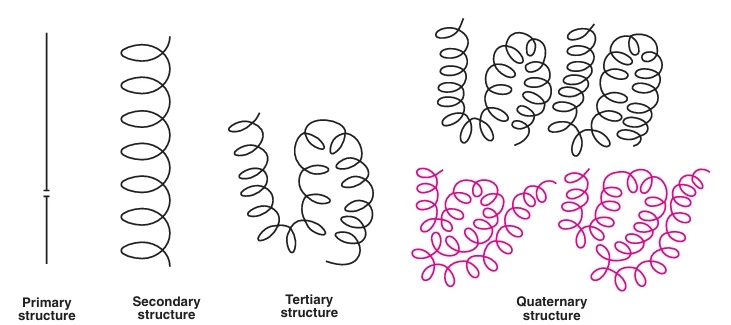 Fig: Structural Organization of proteins
Fig: Structural Organization of proteins
Primary Structure:
- Linear sequence of amino acids
- Held together by peptide bonds
- Backbone of protein
- Responsible for its function
- Each protein has unique sequence,
- Determined by genes.
- Genetic diseases occur changes in primary structure
Peptide Bond
- Amino acids are held together in a protein by covalent peptide bond or linkages.
- Strong bonds and serves as cementing material between individual amino acids.
- Partial double bond in character.
- Both –C=0 and –NH groups of peptide bonds are polar and are involved in Hydrogen bond formation.
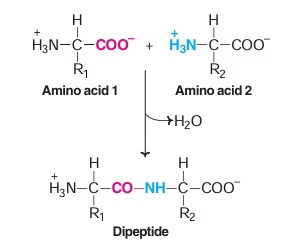 Fig: Peptide bond formation
Fig: Peptide bond formation
Peptide Bond formation
- Amino group of one amino acid combines with carboxyl group of another amino acid.
- Dipeptide: Two amino acids & one peptide bond
- Polypetides: Decapeptides and more.
Secondary Structure:
- The polypeptide chain folded into regular (a-helix, b-sheet) and irregular forms (turns and loops) characterises the secondary structure.
- Collagen: Rich in proline &hydroxyproline
- Forms triple helix
- Stabilized by interchain hydrogen bonds
- α- helix, β-pleated sheet
- Loops & bends, β-turn, Supersecondary structures, Triple helix
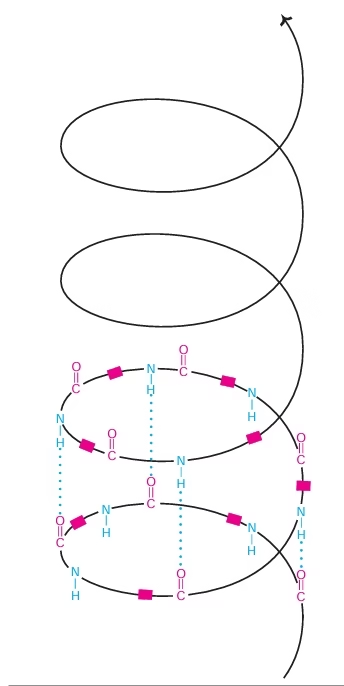 Fig: Alpha helix
Fig: Alpha helix
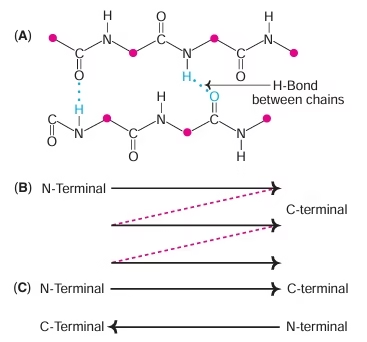 Fig: Beta sheet
Fig: Beta sheet
Tertiary structure:
- Non-linear, 3 dimensional
- Hydrophobic side chains – interiorly
- Hydrophilic side chains – exteriorly
- Formed and stabilized by hydrogen bonds, hydrophobic interactions, electrostatic interactions, disulfide bonds
Domain: Basic unit of protein Structure (tertiary) and function.
Quaternary structure:
- Non-linear
- 3 dimensional
- Global, and across distinct amino acid polymers
- Formed by hydrogen bonding, covalent bonding, hydrophobic, electrostatic interactions.
- Monomer/ Protomer/ Subunit: Individual polypeptide chains.
- Oligomer: two or more polypeptides.
- Oligomeric Proteins: Hemoglobin, Lactate Dehydrogenase (LDH)
- Hemoglobin is tetramer.
Bonds Stabilizing Protein Structure:
- Covalent bonds
- Peptide and disulphide linkages
- Non-covalent bonds
- Hydrogen bonds
- Hydrophobic interactions
- Electrostatic interactions
- Van der Waals interactions
Methods to determine protein structure:
- Determination of amino acid composition in a protein
- Determination of the number of polypeptides in a protein
- Determination of amino acid sequence in a protein
- Sanger’s reagent
- Edman’s reagent
- Automatic sequencing
Methods to explore higher levels of protein structure:
- Electron microscopy
- X-ray crystallography
- Nuclear magnetic resonance (NMR) spectroscopy
Denaturation
- Disorganization of native protein structure.
- Only primary structure remains intact.
- Protein loses biological function & 3-D form.
- Usually irreversible.
Agents of denaturation:
- Physical: Heat, Mechanical shaking, X-rays.
- Chemical: Acids, alkalies, salts of heavy metals.
Coagulation:
- When heated, irreversible denaturation results in coagulation.
- Coagulum is thick, semisolid precipitate of protein.
Plasma Proteins
Plasma
- Clear straw colored fluid derived from blood
- 90% water and 10% solutes
- Mainly albumin, globulins, fibrinogen
Serum
- Defibrinogenated plasma
- Consists only of albumin and globulins
Functions of plasma proteins
- Maintenance of osmotic pressure
-
- Proteins are colloidal
- Albumin contributes to 75-80 % of total plasma osmotic pressure.
- Maintains blood volume and body fluid distribution
- Edema occurs in Kwashiorkor: Decrease in plasma albumin
2. Transport function
-
- Albumin – transport of FFA, Unconjugated bilirubin, Calcium, Copper
- Transferrin – transport of iron
3. Nutritive function
-
- Good source of energy in starvetic state
- Source of amino acids for tissue protein synthesis
- Maintenance of viscosity of blood
-
- Globulins play major role due to large size and asymmetry
- Provide resistance to the flow of blood in blood vessels
- Maintain the blood pressure in normal range
- Immunological function
-
- Gamma globulins provide antibodies
- Protect body against microbial infections
- Blood coagulation
-
- Prothrombin, fibrinogen, enzymes and clotting factors
- Buffering action
-
- Albumin: Maximum buffering capacity.
- Maintain physiologic pH of blood
- Reserve proteins
-
- Used for synthesis of new proteins
Functions of Immunoglobulins:
- IgG: cross the placenta & transfer mother’s imunity to foetus. It triggers foreign cell destruction & responsible for humoral immunity.
- IgA: Secretory antibody which protects the body surface. It is the mainly found in colostrum.
- IgM: Macroglobulins & Natural antibody. Serves as a first line of defense & are very effective for invading microorganisms.
- IgD: function as B-cell receptor.
- IgE: induce histamine release from mast cell & cause allergy.
Digestion and Absorption of Proteins
- The daily protein load handled by the gut is obtained from two sources- about 70-100 g dietary protein and 35-200 g endogenous protein (derived mostly from digestive enzymes and mucus secreted into the gut as a result of the disintegration of epithelial cells).
- The dietary proteins are denatured on cooking. Denaturation makes proteins more susceptible to enzyme activity by the unfolding of the polypeptide chain, and hence cooked proteins are more easily digested.
- The digestion & absorption of proteins in the gut is very efficient process and hence only 1-2 g nitrogen, equivalent to 6-12 g of protein are lost in the feces daily.
- In humans the degradation of ingested proteins into their constituent amino acids occurs in the gastrointestinal tract
- There are no Proteolytic enzymes in mouth
- Site: stomach, small intestine
- Enzymes: pepsin, Proteolytic enzymes of pancreas.
Proteins are broken down by the hydrolysis of peptide bonds and hence the enzymes involved are termed as peptidases, belonging to the class hydrolases.
- Peptidases are of two types:
- Endopeptidase pepsin, trypsin
- Exopeptidases
Exopeptidases are sub classified into carboxypeptidases (act on C-terminal end) and aminopeptidases (act on N-terminal end).
Digestion of Proteins in Stomach:
- Pepsin:
- Proteinase, non specific endopeptidase
- Secreted by chief cells
- Inactive form – pepsinogen
- Works at acidic pH(1.6-2.5)
- Hydrolysis of pepsinogen by HCL or pepsin itself to form active pepsin
- Cleaves peptide bonds of Aromatic amino acids & Acidic amino acids.
- Gastric mucosal cells
Secrete gastrin → Gastric juice release → Contains HCl, Pepsinogen, Rennin in infants, Gastriscin, Gelatinase
- The acidic gastric juice is both an antiseptic and a denaturing agent
- Pepsinogen an inactive precursor or zymogen is converted to pepsin by the enzymatic action of pepsin itself
- In the stomach pepsin hydrolyses ingested proteins at peptide bonds on the amino terminal side of the aromatic amino acid residues Phe, Trp, Tyr cleaving long polypeptide chains into a smaller peptides
Digestion of Proteins in Pancreas:
- Low pH of gastric contents leads to bicarbonate secretion by pancreas
- Amino acids in duodenum release of cholecystokinin
- Trypsinogen → Trypsin (Enterokinase- Autoactivation)
- Chymotrypsinogen ⇒ ⇒ ⇒ ⇒ Chymotrypsin
- Proelastase ⇒ ⇒ ⇒ ⇒ Elastase
- Procarboxypeptidase A & B ⇒ ⇒ ⇒ ⇒ Carboxypeptidase
Digestion of proteins in Intestine:
- Aminopeptidases:
- Non- specific exopeptidases
- Cleaves N-terminal amino acids
- Free amino acids & smaller peptides produced
- Dipeptidases:
- Act on Dipeptides
- Amino acids liberated
Absorption of Amino acids:
- Na+ dependent active transport
- Na+ & amino acid share common carrier
- Energy supplied by ATP
- Similar to transport of D-glucose
- Na+ independent system
- Cytochalasin B inhibits it.
3.ϒ-glutamyl cycle or Meister cycle
- Involves glutathione
- 3 ATP used for transport of single amino acid
- Operative for transport of cysteine & glutamine
Abnormalities of Protein Digestion & Absorption
- Defect in pancreatic secretion due to
- Pancreatitis
- Cystic fibrosis
- Surgical removal of pancreas
- Hartnup’s disease
- Inability to absorb neutral amino acids.
- Tryptophan absorption severely affected.
- Leads to pellagra.
Ammonia
- Ammonia is constantly liberated in the metabolism of amino acids and other nitrogenous compounds.
- Urea is the end product of protein metabolism (amino acid metabolism).
- The nitrogen of amino acids, is converted to ammonia, is toxic to the body.
- It is converted to urea & detoxified.
- As such, urea accounts for 80-90% of nitrogen containing substances excreted in urine.
Metabolic fate of ammonia:
- Formation of glutamate, glutamine & urea
- Synthesis of compounds
-
- Non-essential amino acids
- Purines, pyridines
- Amino sugars
- Aspargine
- NH4 –Important in maintaining acid-base balance of the body
Transport of ammonia:
- Glutamine as a ‘store-house’ of ammonia
- Transport of ammonia through alanine
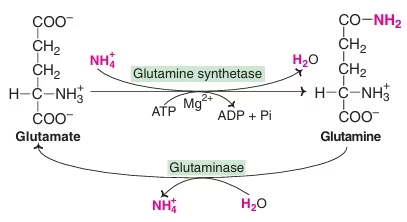 Fig: Synthesis of glutamine and conversion to glutamate
Fig: Synthesis of glutamine and conversion to glutamate
Disposal of Ammonia:
- Ammonotelic Organisms: Most aquatic vertebrates
- Ureotelic organisms: Most terrestrial vertebrates
- Uricotelic organisms: birds, reptiles
Urea
- End product of protein metabolism
- Accounts for 80-90% of nitrogen containing substances in urine
- Synthesized in liver
- Human beings excrete about 10 kg of urea per year
Urea Cycle:
- 1st metabolic cycle elucidated by Hans Krebs & Kurt Henseleit(1932)
- Cyclic process
- Five reactions , five enzymes
- Involves ornithine, citrulline, arginine ,aspartic acid
- Site of synthesis – liver
- Location of enzymes – partly mitochondrial, partly cytosolic
Steps:
- synthesis of carbamoyl-phosphate
- synthesis of citrulline
- synthesis of arginosuccinate
- cleavage of arginosuccinate
- cleavage of arginine to form ornithine & urea
Reactions of urea cycle:
- In the synthesis of urea, one amino group is obtained from the ammonium ion, while the other is contributed by aspartate; carbon atom comes from CO2 (as bicarbonate).
- This is represented in different colours.
- The first two reactions of the cycle occur in the mitochondria. The rest of the cycle occurs in cytosol. (NAG — N-acetyl glutamate)
Significance of urea cycle:
- Detoxification of NH3
- Biosynthesis of arginine
- Disposes off two waste products—NH3 & HCO3
- Participates in blood pH regulation
- Involved in metabolic integration of nitrogen metabolism
- Ornithine—precursor for polyamines & proline
Regulation of urea cycle:
- Feed forward mechanism
– Regulation by substrate availability (ammonia)
– High protein diet& prolonged fasting: induction of urea cycle
- Allosteric regulation
– CPS-I allosteric regulatory enzyme, pacemaker enzyme
– Allosterically activated by N-Acetylglutamate
– High protein diet
– Arginine
– Starvation
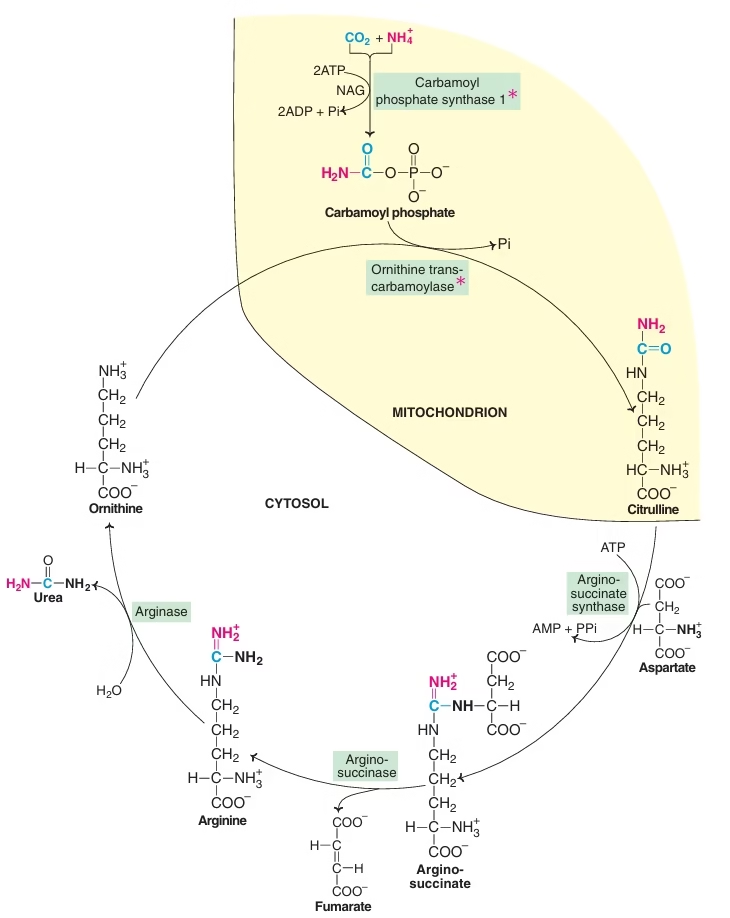 Fig: Urea cycle
Fig: Urea cycle
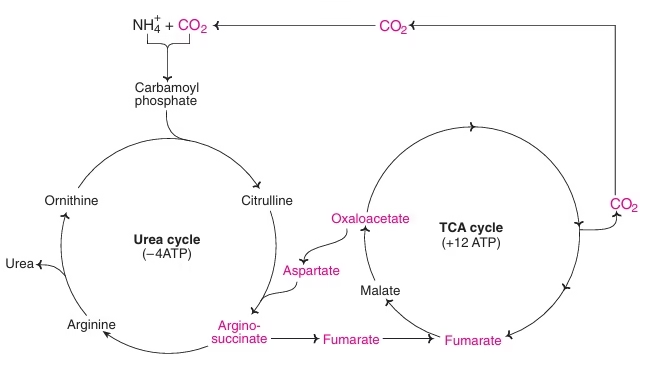 Fig: Metabolic link between urea cycle & TCA cycle
Fig: Metabolic link between urea cycle & TCA cycle
Disorders of Urea Cycle:
| Disorder | Defective enzyme | Product accumulated |
| Hyperammonemia Type 1 | CPS-I | Ammonia |
| Hyperammonemia Type 2 | Ornithine
Transcarbamoylase |
Ammonia |
| Citrullinemia | Arginosuccinate
Synthetase |
Citrulline |
| Arginosuccinic
aciduria |
Arginosuccinate
Lyase |
Arginosuccinate |
| Hyperarginemia | Arginase | Arginine |
Table : Disorders of urea cycle
Blood urea – Clinical Significance:
- Urea levels in the blood are estimated to assess kidney function.
- In healthy individuals, the normal blood urea concentration ranges between 15–40 mg/dl.
- The term Uremia is used to refer to elevated blood urea due to renal disease.
- The conditions in which blood urea levels are elevated are classified into three categories
- Pre-renal
- Severe dehydration, fevers, diabetic coma, thyrotoxicosis, severe burns and so on are pre-renal causes of elevated urea levels.
- Renal
- Blood urea is increased in kidney diseases such as, acute glomerulonephritis, pyelonephritis, polycystic kidney, nephrotic syndrome and so on.
- Post-renal
- When there is an obstruction to the flow of urine (e.g. stones, tumours, and stricture urethra, enlargement of prostate and so on) blood urea is elevated, due to the increased Reabsorption in the renal tubules.
- Blood urea nitrogen (BUN)
BUN = ½ NPN (approx.)
Amino acid metabolism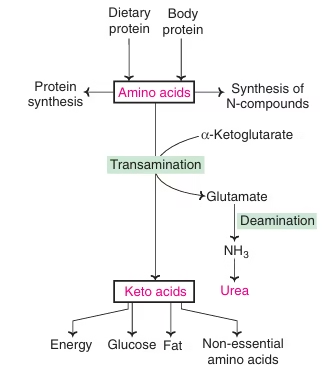
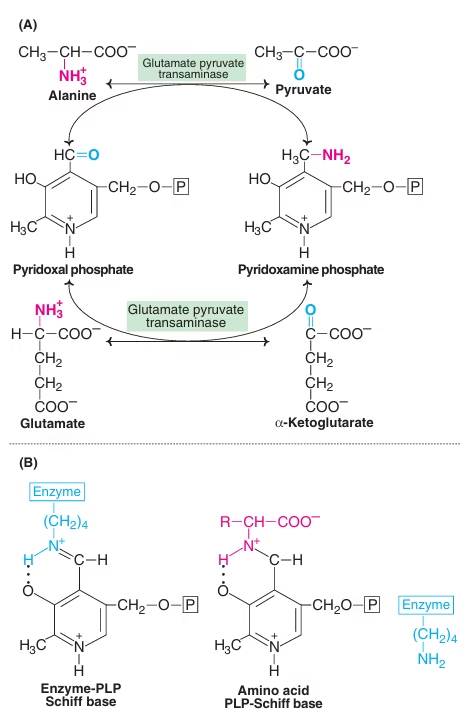
Transamination
- The transfer of an alpha-amino group from an alpha-amino acid to an alpha-keto acid is known as transamination.
- The reaction involves the interconversion of a pair of amino acids and a pair of keto acids.
- The overall process is catalysed by a group of enzymes known as transaminases or aminotransferases.
- The derivative of vitamin B6– pyridoxal phosphate (PLP) acts as the coenzyme.
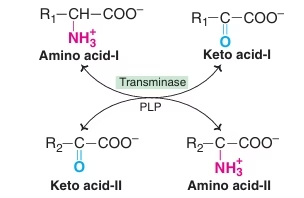 Fig: Transamination reactions
Fig: Transamination reactions
Salient Features of Transamination:
- Reversible reaction
- Intermolecular transfer of NH2 group, no NH3 formation
- Site – liver, kidney , heart ,brain (principally)
- Coenzyme – Pyridoxal phosphate (PLP)
- Production of non-essential amino acids
- Group Transaminases or Aminotransferase –
- Uses any of α-ketoglutarate, Oxaloacetate, pyruvate
- Specific transaminases – ALT or SGPT , AST or SGOT
- Amino acids not participating in Transamination: lysine, threonine, proline, hydroxyproline
- Transamination is not restricted to α-NH2 group.
Deamination
- Process of removal of nitrogen of amino acid as NH3
Oxidative Deamination by glutamate dehydrogenase:
- Glutamate – collection centre for NH2
- Glutamate- Only Amino Acid undergoing Oxidative deamination
- Freely reversible reaction
- Functions in both AA anabolism & AA catabolism
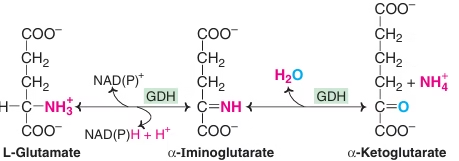
Fig: Oxidative Deamination by glutamate dehydrogenase
Oxidative Deamination by amino acid oxidase:
- L-Amino acid oxidase & D-Amino acid oxidase— flavoproteins
- Possess FMN & FAD respectively
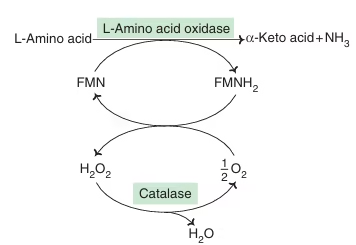
Fig: D-amino acid oxidation
Non-oxidative Deamination:
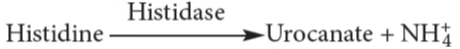
- Hydroxy Amino acids
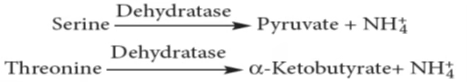
- Sulphur amino acids
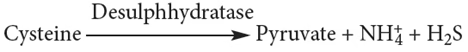
- Deamination of histidine
Transmethylation
- Transfer of methyl group (-CH3) from active Methionine to an acceptor
- Methionine has to be activated to S-adenosyl methionine (SAM) to donate methyl group.
- 3 ATPs are consumed in this SAM formation.
- Enzyme- Methyl transferases
| Methyl group acceptor | Methylated products |
| Norepinephrine | Epinephrine |
| Epinephrine | Metanephrine |
| Nicotinamide | N-methyl nicotinamide |
| Ethanolamine | Choline |
| Phosphatidylethanolamine | Phosphatidylcholine |
| Acetyl serotonin | Melatonin |
| Serine | Choline |
| tRNA bases | Methylated tRNA bases |
| Protein-amino acid residues | Protein-methylated amino acids |
| Norepinephrine | Epinephrine |
| Epinephrine | Metanephrine |
| Nicotinamide | N-methyl nicotinamide |
| Ethanolamine | Choline |
Significance of Transmethylation:
- Many compounds become active only after Transmethylation.
- Protein methylation controls protein turnover. Methylation protects the proteins from degradation.
- SAM is precursor for synthesis of a plant hormone, ethylene, which regulates plant growth and development and involved in fruit ripening.
Decarboxylation
- Some alpha amino acids undergo decarboxylation to form respective amines.
- Enzymes: Decarboxylase dependant on PLP (Pyridoxal Phosphate)
Serotonin (from Tryptophan): Nerve impulse transmission![]()
Histamine (from histidine): Vasodilator & lowers the blood pressure![]()
GABA (from Glutamate): inhibits transmission of nerve impulse.![]()
Catecholamine (dopamine, NE, E) from tyrosine.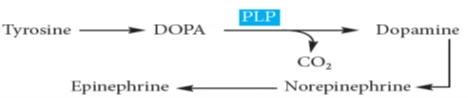
Glycine – Metabolic Products
- Synthesis of Purine ring
-
- Glycine contributes to the C4, C5 and N7 of the purine ring.
- Synthesis of Heme
- Formation of glutathione
-
- Glutamyl-cysteinyl-glycine
- As a neurotransmitter
- Glycine as conjugating agent: (Bile acids)
-
- Cholic acid + Glycine → Glycocholic acid
- Chenodeoxycholic acid + Glycine → Glycochenodeoxycholic acid
- Detoxification:
-
- Glycine + Benzoic acid → Hippuric acid
- Formation of Creatine:
-
- Glycine, arginine, Methionine
- Present in tissues muscle. Brain, blood
- As high energy compound phosphocreatine and as free creatine.
- Creatinine is an anhydride of creatine.
- It is excreted in urine.
Phenylalanine and Tyrosine
- Phe and Tyr are structurally related amino acids.
- Phe: essential; Tyr: nonessential
- Phe & Tyr involved in synthesis of-
- Catecholamine- Epinephrine, Norepinephrine (adrenal medulla)
- Catecholamines- dopamine (CNS)
- Thyroid hormones (Thyroid gland)
- Melanin pigment (Skin, hair, eyes)
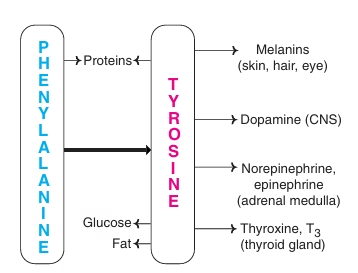
Fig: Metabolic products of Phenylalanine and tyrosine
Role of melanin;
The colour of skin and hair is controlled by large number of genes. In humans, melanin is the primary determinant of skin color. The most important function of melanin is the protection of the body from sun radiation.
Role of Catecholamines:
- Dopamine & Norepinephrine serve as neurotransmitters in the brain & ANS.
- Norepinephrine & Epinephrine regulate carbohydrate & lipid metabolisms. They stimulate degradation of triacylglycerol & glycogen.
- They cause increase in blood pressure.
Conversion of Phenylalanine to Tyrosine:
- Irreversible reaction
- Require coenzyme-biopterin
- Active form is tetrahydrobiopterin(BH4)
- Tetrahydrobiopterin (BH4) is oxidised to dihydrobiopterin & then regenerated by NADPH dependent enzyme dihydrobiopterin reductase.
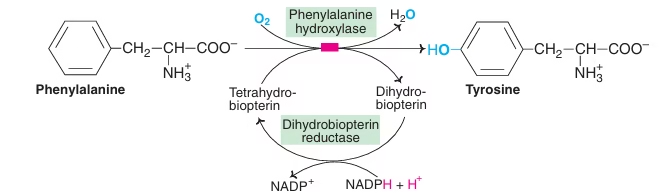
Fig: Conversion of Phenylalanine to tyrosine
Phenylketonuria (PKU)
- Most common disorder of Amino Acid metabolism occurs in 1:10000 live births. (Autosomal Recessive)
- Enzyme defect: Deficiency of enzyme Phenylalanine hydroxylase.
- Primarily causes accumulation of Phenylalanine in tissues & blood and its increase excretion in urine.
- Phenylalanine levels are elevated in plasma upto 20-70 mg/dl (Normal 1-2 mg/dl)
- Altered Metabolism: Phenylalanine is diverted to alternate pathway results in excessive production of phenylpyruvate, phenylacetate, phenyllactate & phenylglutamine. The name of the disease, phenylketonuria is derived from the fact that large quantities of phenylpyruvate which is a keto acid (phenylketone) are excreted in urine.
- Clinical features:
- Accumulation of phenylalanine and its metabolites impairs the normal development of brain in the newborn causing mental retardation, severe neurological deficits and very low IQ.
- High frequency of epilepsy is seen in phenylketonurics.
- Growth is adversely affected and the patient presents with abnormal gait.
- Patients of PKU, as they cannot form tyrosine, exhibit light colour of skin, hair and eyes due to under pigmentation.
- Diagnosis:
- Screening of neonates for increased level of Phe in blood (20-70 mg/dl) carried out by Guthrie test which is bacterial bioassay for Phe.
- In urine by Ferric chloride test.
- With early recognition of PKU, mental retardation can be avoided.
- Treatment:
- Limit phenylalanine intake & tyrosine should be given in diet as it becomes essential amino acid in this condition.
- The special diet, mostly synthetic diet low in phenylalanine, but containing adequate amounts of tyrosine should be continued till the child reaches 5 years of age to prevent damage to the brain.
- Proteins with a low phenylalanine content, such as casein from milk, are hydrolysed and phenylalanine is removed making it suitable for phenylketonurics.
Alkaptonuria
- Autosomal Recessive
- 1 in 25000
- Enzyme Defect: Homogentisate oxidase in tyrosine metabolism
- Homogentisate accumulate in tissue & blood and excreted in urine.
- Homogentisate, on standing, gets oxidised to corresponding quinones which polymerises to give black or brown colour. So, urine of alkaptonuria patient resembles coke in colour. Black Urine Disease.
- Biochemical changes: Homogentisate gets oxidized to benzoquinone acetate which is polymerized to a black colored pigment, alkapton. Alkapton is deposited in bones, connective tissue and other organs (nose, pinna of the ear) causing a condition known as ochronosis. These deposits may be responsible for the occurrence of arthritis in the fourth and fifth decades of life.
- Clinical Features: Asymptomatic in childhood, Tendency toward arthritis in adulthood.
- Diagnosis: Change in colour of urine on standing to brown or dark.
- Urine gives positive test with ferric chloride & silver nitrate due to strong reducing activity of homogentisate.
- Treatment- Does not require specific treatment, however low Phenylalanine diet is recommended. High dose of ascorbic acid are used to stall the pigment deposition on collagen.
Tryptophan metabolism
- Trp was first to be identified as an essential AA.
- Both glucogenic & ketogenic
- Precursor for the synthesis of
- NAD+ & NADP+ (coenzymes of niacin)
- Serotonin & melatonin
Serotonin:
- Potent vasoconstrictor and causes smooth muscle contraction in arterioles and bronchioles.
- Excitatory neurotransmitter in the brain.
- Causes peristalsis and causes the release of peptide hormones from GI tract.
- Influencing the behavioural patterns, sleep, blood pressure, body temperature in humans.
Melatonin:
- Hormone synthesized by pineal gland.
- Involved in circadian rhythm or diurnal variations (24 hr cyclic process in the body).
- Neurotransmitter
- Inhibits production of MSH and ACTH.