Definition of Lipids
Organic substances that are relatively insoluble in water, soluble in nonpolar organic solvents (alcohol, ether, benzene, chloroform, etc.), are actually or potentially related to fatty acids and utilised by the living cells.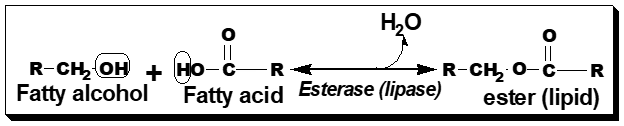
Properties of Lipids
- Not true polymers; unlike polysaccharides, proteins & nucleic acids.
- Mixture of a large number of unrelated substances.
- Not macromolecules; smaller in size.
- Non-polar (Predominantly composed of carbon and hydrogen atoms linked together through neutral covalent bonds)
Functions of Lipids
- Fuel reserve: TAG in adipose tissue
- Components of Membrane structure and membrane permeability
- Source of fat soluble vitamins
- Cellular metabolic regulators eg. steroid hormones, prostaglandins (local hormones)
- Insulation to internal organs against changes in external temperature. (Thermal insulators)
- Propagation of Nerve impulse (Electrical insulators).
- Participates in ETC in cellular respiration.
Classification of Lipids: Bloor’s classification
- 1. Simple lipids
- 2. Complex or Compound lipids
- 3. Derived lipids
- Miscellaneous lipids
- Simple lipids: Esters of fatty acids with alcohol.
a. Fats and oils( Triacylglycerol) –
Esters of FA with glycerol.
Oil- Liquid; Fat- Solid (at room temp)
b. Waxes-
Esters of FA (long chain) with alcohol other than glycerol.
Skin glands of certain vertebrates secrete waxes to protect hair and skin.
- Complex or Compound Lipids:
- Esters of fatty acids with alcohol containing additional groups such as phosphate, nitrogenous base, carbohydrate, protein etc.
- a) Phospholipids:- Contains phosphoric acid and nitrogenous base.
- i) Glycerophospholipids :- e.g. Lecithin, cephalin
- ii) Sphingophospholipids :- e.g. Sphingomyelin
- b) Glycolipids: These lipids contain a fatty acid, carbohydrate and nitrogenous base. The alcohol is sphingosine , hence they are also called as glycosphingolipids. Eg. Cerebrosides, Gangliosides
- c) Lipoprotein:- Macromolecular complexes of lipid with proteins.
- d) Other complex lipids:- Sulfolipids, Aminolipids & Lipo-polysaccharide.
- Derived Lipids:
- These are the derivatives obtained on the hydrolysis of simple and complex lipids.
- These include glycerol and other alcohols, fatty acids, mono and diacylglycerol.
- Lipid soluble vitamins, steroid hormones, ketone bodies, bile acids, cholesterol.
- Miscellaneous Lipids
Compounds possessing the characteristic of lipids:
- Carotenoids,
- Squalene,
- Hydrocarbons,
Phospholipids
Structure:
Phospholipids are composed of:
- Fatty acids (a saturated and an unsaturated fatty acid).
- Nitrogenous base (choline, serine, threonine, or ethanolamine).
- Phosphoric acid.
- Fatty alcohols (glycerol, inositol or sphingosine).
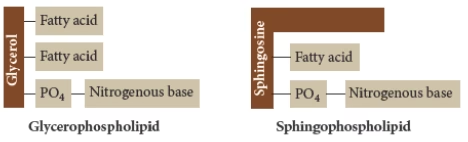
Phospholipids are divided into two classes based on the type of alcohol present:
- Glycerophospholipids: They contain alcohol glycerol (C3)
- Sphingophospholipids: They contain alcohol sphingosine (C18)
Glycerophospholipids:
Major lipids in biological membranes.
- Phosphatidic acid: Simplest phospholipid.
It is an intermediate in the synthesis of triacylglycerol and phospholipids.
- Lecithins- Phosphatidylcholine
The most abundant group of phospholipids in the cell membranes.
Dipalmitoyl lecithin:-
- It is found in lung.
- Surface active agent and prevents the adherence of inner surface of the lungs due to surface tension.
- Deficiency causes- Respiratory distress syndrome in premature infants.
- Cephalins: Phosphatidyl-ethanolamine
- It plays a role in blood coagulation.
- Phosphatidylserine
- Phosphatidylinositol:
- Important component of cell membranes.
- It acts as a second messenger for the action of certain hormones
- Plasmalogen:
- Similar to other phospholipids but the fatty acid chain at C1of glycerol is linked through an ETHER rather than ester bond
- There are three major classes
- phosphatidalcholines
- phosphatidalethanolamines
- phosphatidalserines
- These are found in myelin and cardiac muscle
- Platelet activating factor (PAF) is a plasmalogen and involved in platelet aggregation and degranulation
- Cardiolipins:
- It is the only phosphoglyceride that posses antigenic property
Functions of phospholipids:
- Form the structural components of membranes and regulate membrane permeability
- Lecithin , cephalin, and cardiolipin in mitochondria are responsible for maintaining the conformation of ETC components, and cellular respiration
- They participate in absorption of fat from intestines.
- Essential for the synthesis of lipoproteins and thus participate in transport of lipids.
- Accumulation of fat in liver can be prevented hence they are regarded as lipotropic factors.
- Production of eicosanoids (prostaglandins, prostacyclins , thromboxanes etc)
- Removal of cholesterol from body by reverse cholesterol transport
- Acts as a surfactants
- Cephalins are useful in blood clotting
- Signal transmission across membranes
Lipoproteins
- They are the molecular complexes of lipids with proteins.
- They are the transport vehicle for lipids in circulation.
- They are of five types
- Plasma lipoproteins are separated by electrophoresis into different fractions
- Chylomicrons
- Very low density lipoproteins VLDL
- Low density lipoproteins LDL
- High density lipoproteins HDL
- Free fatty acid-albumin complexes
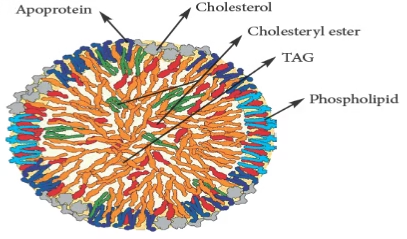 Fig: Lipoproteins (Note the hydrophilic exterior and hydrophobic interior)
Fig: Lipoproteins (Note the hydrophilic exterior and hydrophobic interior)
Characteristics of Lipoproteins:
- Chylomicrons: Synthesized in the intestinal mucosal cells. Contains mostly TAG.
- VLDL: Synthesized in the liver. Converted to IDL and finally to LDL.
- LDL: Derived from VLDL in circulation.
- Elevated levels are associated with vascular diseases.
- HDL: Synthesized in liver and serve as repository of certain apoproteins.
Functions of Lipoproteins:
- Chylomicrons: Transport exogenous TAG to various tissues.
- VLDL: Transport endogenous TAG to various tissues.
- LDL: Transport cholesterol from liver to other extrahepatic tissues: Lethally Dangerous Lipoprotein.
- HDL: Transport cholesterol from extrahepatic tissues to liver. (Reverse cholesterol transport): Highly desirable Lipoprotein
Apolipoproteins/ Apoproteins:
- These are the protein components of lipoproteins.
- Four major types:
-apo A
-apo B
-apo C
-apo E
Role of apolipoproteins:
- Maintaining structural integrity of lipoproteins
- Recognize the cell membrane surface receptors.
- Activate enzymes involved in lipoprotein metabolism.
Eicosanoids
- Definition: Group of biologically active molecules synthesized from eicosaenoic acids (C20 PUFA). Act as local hormones.
- Synthesized from Arachidonic acid.
- Prostaglandins (PG)
- Prostacyclins (PI)
- Thromboxanes (TX)
- Leukotrienes (LT)
- Lipoxins (LX)
Biological role and Therapeutic applications of Prostaglandins and eicosanoids:
- Inflammation:
Tissue response to injury is characterized by 5 cardinal signs- dolour (pain), calor (raised temp.), rubor (redness), tumor (swelling) and loss of function. .
Eicosanoids PGE1 & PGE2 are the natural mediators of inflammation. This is the basis of the use of corticosteroids and various non-steroidal antiinflammatory drugs (NSAID) (which inhibit the eicosanoid synthesis) in the treatment of various inflammatory disorders (e.g. rheumatoid arthritis).
Corticosteroids block the enzyme Phospholipase, while Aspirin is an irreversible inhibitor of Cyclooxygenase.
- Regulation of Blood Pressure
Prostaglandins act as vasodilators. Hence, they are used in the treatment of hypertension.
- Effects on platelet aggregation:
Thromboxanes cause platelet aggregation forming blood clot.
Prostacyclins inhibit platelet aggregations.
- Effects on reproduction:
Prostaglandins PGE2 & PGF2 are used in medical termination of pregnancy, induction of labour and arresting postpartum hemorrhage.
- Effect on GIT:
PGE increases intestinal motility and inhibits gastric acid secretion,
PGE used in treatment of gastric ulcers.
- Effects on respiratory system:
PGE- Acts as a bronchodilator; PGF- acts as a bronchoconstrictor.
PGE useful in treatment of bronchial asthma.
- Effects on metabolism:
PG influences metabolic reactions through mediation of cAMP. PGE decreases lipolysis, increases glycogenesis and mobilization of calcium from bone.
- Effects on excretory system:
Increases GFR and promotes urine output.
- Leukotrienes:
Leukotrienes are Integral component of SRS-A (slow reacting substance of anaphylaxis).
Overproduction causes asthmatic attacks.
Essential Fatty Acids
- The fatty acids that are required by humans, but are not synthesised in the body and hence need to be supplied in the diet.
- Linoleic acid (18: 2; 9, 12)
- Linolenic acid (18: 3; 9, 12, 15)
- Arachidonic acid (20: 4; 5, 8, 11, 14) is considered semi essential since it can be synthesised from linoleic acid (note that both belong to ω6 series).
- Only linoleic acid and linolenic acid are essential because humans lack the enzymes that can introduce double bonds beyond carbon no. 9.
- Vegetable oils are the richest source of fatty acids.
- Oils rich in SFA: Palm oil, coconut oil, ghee.
- Oils rich in MUFA: Groundnut oil, gingily oil, mustard oil, sesame oil.
- Oils rich in PUFA: Sunflower oil, cotton seed oil, safflower oil, rice bran oil, soyabean oil, linseed oil, corn oil.
Functions of EFA:
- Integral components of membrane structure.
- Concentrated source of energy
- Formation of lipoproteins: Transport of cholesterol
- Prevention of fatty liver (lipotropic factors)
- Formation of Eicosanoids.
- Bioavailability of Fat soluble vitamins.
- Consumption of EFA (ω3 series: Linolenic acid) lowers the risk of cardiovascular disease.
Deficiency of EFA:
- Rare in humans.
- In infants receiving formula diet and patients on IV nutrition low in EFA.
- Phrynoderma or toad skin or Scaly dermatitis
- Characterized by presence of horny eruption on the posterior and lateral parts of limbs ,back and buttocks.
Digestion & absorption of Lipids
Digestion of Lipids:
- Poor water solubility of lipids: not easily accessible to digestive enzymes.
- Digested to only small extent in mouth.
- Digestion of short and medium-chain TG begins in the as stomach via action of gastric lipases (pH 5.4). Important in infants as mother’s milk contains predominantly SC & MC FAs.
- In adults, diet contains mainly long-chain FA- TG; digestion occurs exclusively in the small intestine.
Emulsification in small intestine:
- Dispersion of lipids into smaller emulsion droplets due to reduction in surface tension.
- Increases the surface area of the lipid droplets.
- Essential for effective digestion of lipids.
- Enzymes (lipases) act at the interfacial area between aqueous & lipid phase.
Three Mechanisms of Emulsification:
- Bile salts–secreted in bile by liver; most effective biological emulsifying agents.
Sodium and potassium salts of glycocholic or taurocholic acid.
- Surfactant action of degraded lipids (FFA & MAG) along with phospholipids- Surfactants Increase the interfacial area of lipid droplets.
- Mechanical mixing due to peristalsis
Digestion by pancreatic enzymes:
- Pancreatic lipase converts (pH 8.2) triacylglycerols into Monoacylglycerols and fatty acids.
- Cholesterol esters are hydrolysed by pancreatic cholesteryl esterase.
- Phospholipids are degraded by action of pancreatic phospholipase A2 and then by lysophospholipase.
- Monoacylglycerols hydrolysed by lipid esterase to glycerol and fatty acids.
Three Important lipases in lipids digestion:
| GIT Organ | Enzyme | Stimulators |
| 1.Stomach | Gastric lipase (pH 5.4)
Only SC& MC-FA digested, Important in children |
|
| 2.Intestine | Pancreatic lipase(pH 8.2)
Phospholipase A2, Cholesteryl Esterase LC-FA digested from TAG |
Bile salts-
emulsification |
| 3.Lymphatic system | Lipoprotein lipase (pH 7.4) | Apo-CII & Apo-E derived from HDL |
Bile salts in lipid absorption:
- Form mixed micelles with lipids.
- Smaller in size than lipid emulsion droplets.
- Disc like shape with lipids at the interior & bile salts at the periphery.
- Hydrophobic groups of lipids on the inside and hydrophilic groups on the outside.
- Bile salt micelles exert solubilizing effect on the lipids.
- Vehicles for lipid transport from intestinal lumen to the membrane of intestinal mucosal cells.
Absorption of Lipids into enterocytes
- i) Directly by hepatic portal vein → Liver in bound form to albumin
- – Glycerol – SC & MC FA
- ii) Indirectly: LC-FA in the form of Chylomicrons by lymphatic system.
- Lipid absorption occurs at the brush border membrane of intestinal mucosal cell (enterocyte).
Formation of Chylomicrons:
- Products of lipase action are taken up by the intestinal mucosal cell (enterocyte) and reconverted back to triacylglycerol.
- The newly- synthesised TAG and cholesterol esters are hydrophobic and hence aggregate in aqueous environment (center).
- They are packaged into small particles called chylomicrons – lipid droplets surrounded by thin layer of protein-Apo B48, phospholipid and unesterified cholesterol.
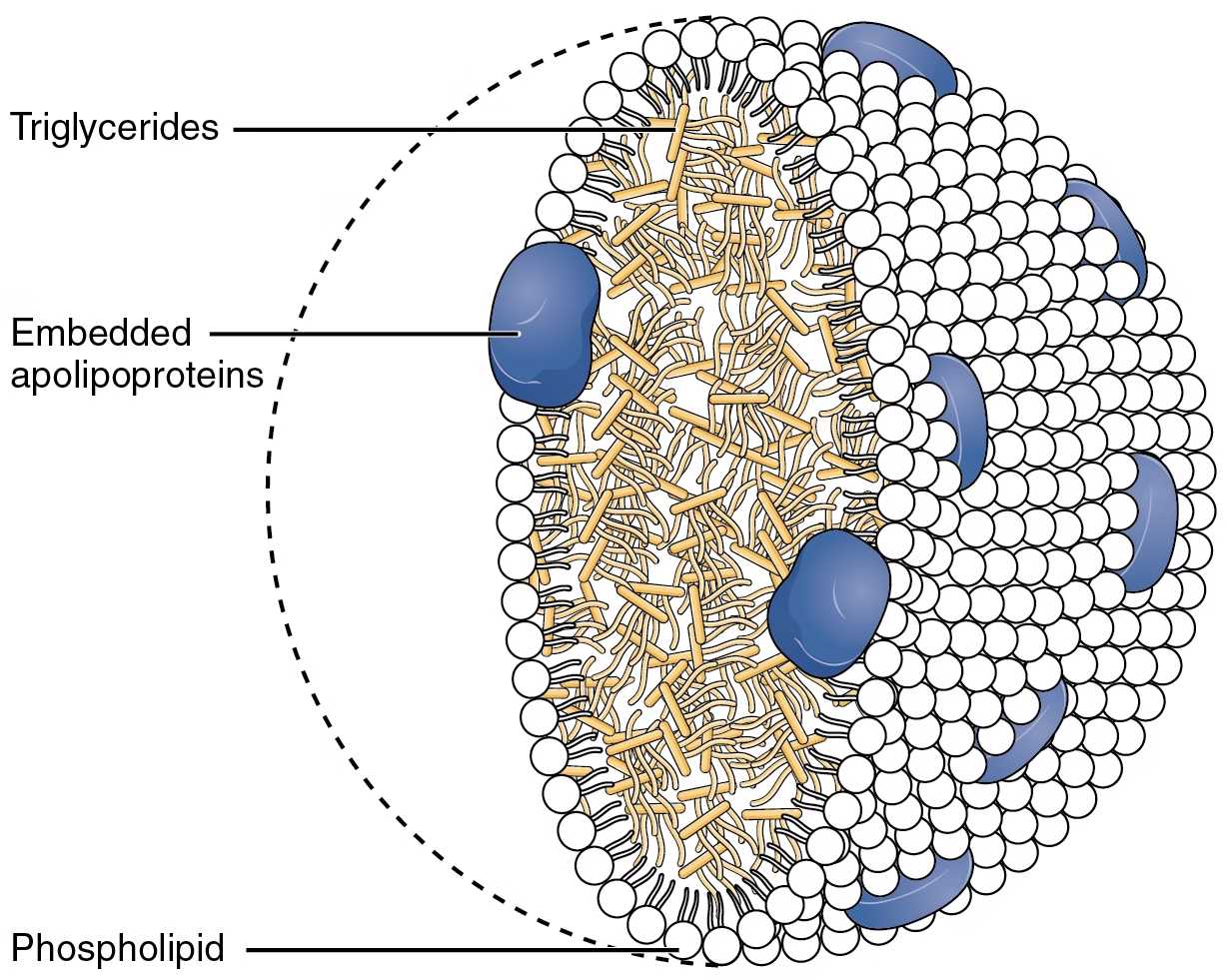 Fig: Chylomicrons
Fig: Chylomicrons
Secretion of lipids:
- Chylomicrons are released from the enterocytes into the lymphatic system and then into the blood stream.
- Blood from here flows to the heart and then to peripheral tissues (muscle, adipose tissue), and finally to the liver.
- In muscles, fatty acids are oxidized for energy; in adipose tissue, re-esterified for storage as triacylglycerol.
Abnormal digestion and absorption of lipids:
- Steatorrhea-
Excretion of fats in feces.
Defect in secretion of bile or pancreatic juice into intestine.
Impairment in lipid absorption by intestinal cells.
- Billiary obstruction- Gallstones, tumour
- Chyluria- Defective urinary connection with the lymphatic system.
Fatty acid oxidation
- Oxidation of fatty acids on β-carbon atom: β-oxidation
- Fatty acids are oxidized by most of the tissues in the body except:
- Brain,
- Erythrocytes
- Adrenal medulla.
Nomenclature of FA:
- FA: long chain of hydrocarbons with methyl group on one end and carboxyl group on the other.
- Numbering of Carbon atoms:
- Carboxyl carbon : Number 1
- Carbon next to the carboxyl carbon: α
7 6 5 4 3 2 1
- CH3-CH2-CH2-CH2-CH2-CH2-COOH
β α
Stages: FA-oxidation
- 1) Activation of FA occurring in the cytosol.
- 2) Transport of FA into mitochondria.
- 3) β-oxidation proper in the mitochondrial
- Activation of FA occurring in the cytosol:
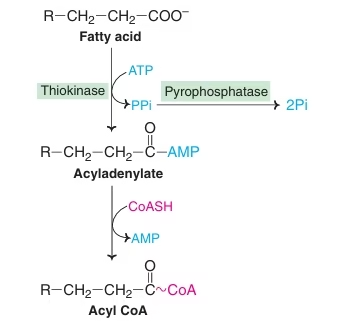 Fatty acid is converted to activated fatty acid (acyl CoA) with the help of enzyme Thiokinase.
Fatty acid is converted to activated fatty acid (acyl CoA) with the help of enzyme Thiokinase.
- Transport of FA into mitochondria:
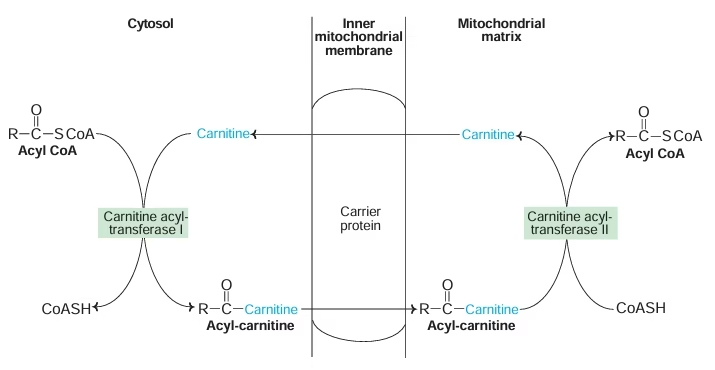 Activated fatty acid (Acyl CoA) is transported from cytosol to mitochondria with the help of carrier protein carnitine located on inner mitochondrial membrane.
Activated fatty acid (Acyl CoA) is transported from cytosol to mitochondria with the help of carrier protein carnitine located on inner mitochondrial membrane.
- β-oxidation proper in the mitochondrial matrix:
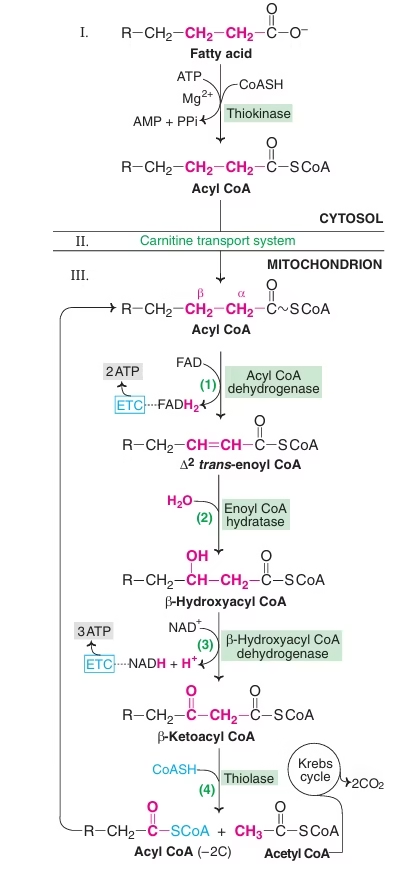 It involves four steps:
It involves four steps:
-
- Oxidation (FADH2 generated)
- Hydration
- Oxidation (NADH2 generated)
- Cleavage
Acyl CoA dehydrogenase is the rate limiting enzyme of this pathway.
Energetics of palmitic acid oxidation:
| Mechanism | ATP yield |
| 1. B-oxidation 7 cycles | |
| 7 FADH2 | 10.5 |
| 7 NADH | 17.5 |
| 2. From 8 Acetyl CoA | |
| Each Acetyl CoA: 10 ATP | 80 |
| Total energy from One Mole Palmitate | 108 |
Alfa- and omega- fatty acid oxidation:
- Occurs in microsomes.
- No energy is produced.
- Peroxisomal oxidation: No ATP is synthesized; heat is liberated.
- Alpha oxidation Example: Phytanic acid (chlorophyll –plant foods, vegetables and ruminant’s milk)
Oxidation of Unsaturated FA
- Provides less energy than that of oxidation of saturated FA.
- Presence of double bonds poses problems for oxidation to proceed.
- Two additional enzymes: Isomerase & Epimerase.
Disorders of Fatty Acid Oxidation:
SIDS- Sudden infant death syndrome
- Deficiency of medium chain acyl CoA dehydrogenase.
- Few hours after feeding, glucose level and its utilization decrease and FA oxidation must increase to meet the energy needs.
Jamaican vomiting sickness
- By eating unripe ackee fruit, containing toxic AA hypoglycin A.
- Inhibits enzyme acyl CoA dehydrogenase.
Peroxisome biogenesis disorders PBDs
- Long chain fatty acids (C26- C38) are not oxidized, accumulate in tissues.
- Zellweger syndrome (Cerebrohepatorenal syndrome) : absence of peroxisomes
- Adrenoleukodystrophy: deficiency of Peroxisomal matrix protein
- Refsum’s disease: deficiency of enzyme phytanic acid α–oxidase (α–oxidation)
Ketone bodies
Ketone Bodies:
- Acetone, Acetoacetate :True ketones
- β- hydroxybutyrate : No keto group.
- Energy yielding; Exception: acetone – cannot be metabolized (volatilized in lungs)
- Ketone bodies are water soluble, so easily transported.
Ketogenesis:
- Synthesis of ketone bodies
- Exclusively in Liver- Mitochondrial matrix.
- Acetyl CoA (from Beta Oxidation of Fatty Acids) as precursor.
- HMG CoA synthase is the rate limiting step.
- HMG CoA synthase is common to cholesterol synthesis and ketone body synthesis.
- Primary KB: Acetoacetate
- Secondary KB: Acetone, Betahydroxybutyrate
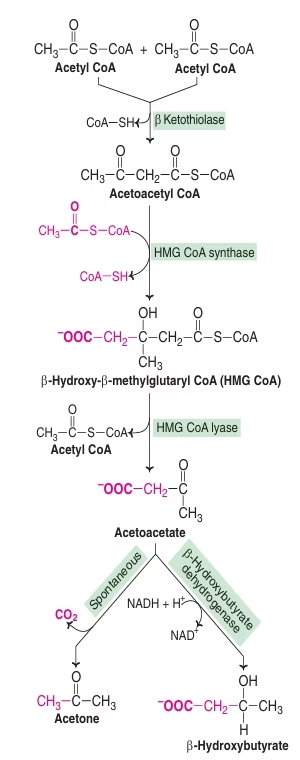 Fig: Ketogenesis
Fig: Ketogenesis
Ketone body Utilization / Ketolysis:
- Source of energy for peripheral tissues- Skeletal muscle, Cardiac muscle, renal cortex.
- Ketone bodies serve as a fuel for extrahepatic tissues.
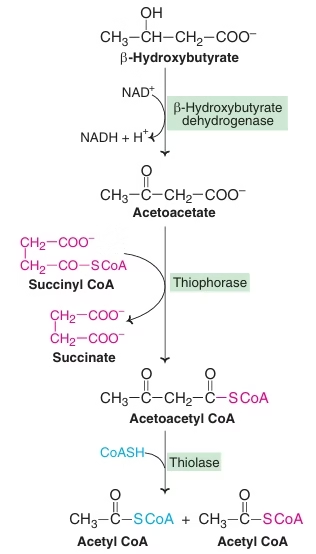 Fig: Degradation of ketone bodies to Acetyl CoA
Fig: Degradation of ketone bodies to Acetyl CoA
Almost all organs utilize KB except:
- Liver: Thiophorase (β- ketoacyl CoA transferase) : Absent in Liver: KB not utilized.
- Erythrocytes- lack mitochondria- cannot utilize ketone bodies.
- More pronounced when glucose is not available as a fuel source as in the case of Starvation & Diabetes mellitus.
- In prolonged starvation: Ketone bodies became the major fuel source for the brain & CNS (50-70 % of Brain’s energy needs). This is an adaptation for survival of organism.
- Brain has an obligatory requirement for glucose. Fatty Acids cannot cross Brain Blood Barrier. Brain adapts to utilize KB during starvation and Diabetes mellitus.
Tests for KB in Urine:
- Rothera’s Nitroprusside test:
Answered by Acetoacetate and acetone.
- Gerhardt’s Ferric Chloride Test:
Answered by Acetoacetate
- KB that cannot be detected by both Tests:
β- hydroxybutyrate
Ketosis:
- In normal persons: Concentration of KB in blood is very low and Excretion of Ketone Bodies in urine is negligible.
- Rate of synthesis of KB in liver exceed their rate of utilization by extrahepatic tissues.
- Ketonemia: Concentration of ketone bodies in Blood elevates.
- Ketonuria : Excretion of ketone bodies in urine
- Ketosis: Ketonemia + Ketonuria
- Smell of acetone in breath, Fruity odour to urine
- Prolonged Starvation & uncontrolled DM- main causes
- Carbohydrate is either unavailable (starvation) or underutilized (diabetes)- Oxaloacetate is unavailable for combining with acetyl CoA Impaired TCA cycle- Acetyl CoA is diverted for KB synthesis.
Diabetic Ketoacidosis:
- Acetoacetate & β- hydroxybutyrate: strong acids.
- Increase in their blood concentration, lowers the blood pH, leading to condition of Metabolic acidosis called as Diabetic ketoacidosis (DKA).
- Hyperglycemia, ketosis, metabolic acidosis, severe dehydration, electrolyte loss.
Regulation of Ketogenesis:
- Due to non-availability of carbohydrates.
- Excessive FA utilization to meet energy requirements of the cells.
- Glucagon- stimulates ketogenesis;
- Insulin- inhibits ketogenesis.
- Diabetes Mellitus: Increased Glucagon/ Insulin ratio- Stimulates ketogenesis.
- Starvation : Increased fatty acid degradation
Atherosclerosis
- Thickening or hardening of arteries due to accumulation of lipids (cholesterol; free & esterified), collagen, fibrous tissue, proteoglycans, calcium deposits etc.
- Progressive disorder that narrows and ultimately blocks the arteries.
- Infarction: stoppage of blood flow resulting in death of affected tissue.
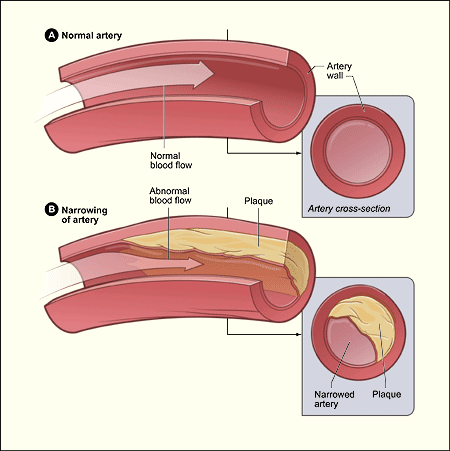 Fig: Atherosclerosis
Fig: Atherosclerosis
Atherosclerosis:
- Coronary arteries: most commonly.
- Coronary Heart Disease CHD.
- Higher in developed countries.
- Risk directly correlated with plasma cholesterol & LDL-C.
- Inversely correlated with HDL-C.
- LDL: Lethally Dangerous Lipoprotein.
- HDL: Highly desirable Lipoprotein.
Risk Factors in Atherosclerosis:
- Major Constitutional risk factors:
- Age iii. Genetic factors
- Sex iv. Familial and racial factors
- Major Acquired risk factors:
- Hyperlipidemia
- Hypertension
- Diabetes mellitus
- Smoking
- Hyperhomocysteinemia
Diseases associated with Atherosclerosis:
Causes of Hypercholesterolemia:
- Diabetes mellitus
- Hyperlipoproteinemias
- Nephrotic Syndrome
- Obstructive jaundice
HDL and Coronary Heart Disease (CHD) :
- Women: Higher HDL: Estrogen: Less CHD.
- Strenous physical exercise, consumption of unsaturated FA, reduction of body weight: Increase HDL: Reduces risk of cardiovascular diseases.
Lipoprotein a Lp (a) : LDL + apo-a
- Inhibits fibrinolysis, reduces breakdown of blood clots and triggers heart attacks. 30mg/dl in plasma: Increased risk of CHD
Control of Hypercholesterolemia/ Prevention of Cardiovascular Disease:
Dietary Advice:
- 1. Consumption of PUFA (Omega 3 FA reduce LDL-C)
- 2. Avoid high carbohydrate (refined) diet
Sucrose steeply raises triacylglycerol levels.
- 3. Avoid cholesterol rich diet:
Restrict overall fat intake : < 20%
Restrict SFA & Trans FA : < 10%
Restrict Cholesterol: < 200 mg/day.
- Dietary fiber: Decrease the absorption of dietary cholesterol
- Consume sufficient amounts of Vitamin C, E, Beta carotene: Natural antioxidants- prevent formation of oxidised LDL.
- Moderate alcohol consumption : Red wine
Lifestyle modification
- Avoid smoking: Nicotine increase lipolysis- elevates acetyl CoA- Increased cholesterol synthesis
- Regular physical exercise:
Reduces LDL-C & TAG and increases HDL-C
Potentiate insulin action and increase activity of lipoprotein lipase.
- Drugs: Statins, Fibrates
Cholesterol
(C27H46O):
- Made up of steroid nucleus: Cyclopentano-perhydrophenenthrene ring
- Major components of cell membrane and lipoproteins
- Exclusively found in animals.
- Amphipathic in nature
Functions of cholesterol:
- Insulating cover for the transmission of electrical impulses in nervous system.
- Role in membrane structure and function.
- Essential ingredient in structure of lipoproteins.
- Synthesis of bile acids, Steroid hormones (sex hormones, cortical hormones) , vit D3.
Serum cholesterol:
- Healthy adults: 140-200 mg/dl.
- Newborn: <100 mg/dl ;
- Rises to 100mg/dl within a year.
- Women of reproductive age have relatively lower plasma cholesterol due to estrogen.
- Cholesterol level increases with increasing age (women after menopause) & also in pregnancy.
Hypercholesterolemia:
Increase in plasma cholesterol >200 mg/dl
Observed in-
- Diabetes mellitus.
- Hypothyoroidism & hypopituitarism
- Obstructive jaundice
- Nephrotic syndrome
- Xanthomatosis
Hypercholesterolemia is associated with atherosclerosis & coronary heart disease.
Hypocholesterolemia:
- 1. Thyrotoxicosis
- 2. Pernicious anemia
- 3. Malabsorption syndrome
- 4. Hemolytic jaundice
- Wasting diseases
Lipid Profile
- Measurement of serum cholesterol alone is not sufficient to assess the risk for cardiovascular disease.
- Other lipid parameters in plasma are equally important.
- Measuring relative concentrations of various lipid fractions in plasma gives comprehensive assessment of cardiovascular disease.
- To estimate serum lipid profile, a sample of serum should be taken after 12 hours of fasting
| Lipid profile parameters | Reference Range (mg/dl) |
| Total Lipid | 400-600 |
| Total Cholesterol | 150-200 |
| LDL-C | 80-150 |
| HDL-C | 30-60 |
| VLDL-C | 20-40 |
| Triglycerides | 75-150 |
Fatty Liver:
- Triacylglycerol accumulate excessively in liver.
- Droplets of TAG – Entire cytoplasm of hepatic cells: Impairment in metabolic functions.
- Associated with fibrotic changes and cirrhosis.
Two main causes:
- Increased synthesis of TAG.
- Impairment in lipoprotein synthesis.
Increased Triacylglycerol (TAG) synthesis:
1) Diabetes mellitus, starvation, high fat diet.
- Increased mobilization of FFA from adipose tissue and their influx into liver is much higher than utilization.
2) Alcoholism
- Inhibits FA oxidation and promotes lipogenesis.
Impairment in lipoprotein VLDL synthesis:
- Defect in Phospholipid synthesis.
- Deficiency of Lipotropic factors.
- Deficiency of EFA.
- Excessive Cholesterol consumption.
- Block in Apoprotein formation.
- Chemicals- Puromycin, Ethionine, CCl4, Lead, Phosphorus
- Failure in the Formation / Secretion of lipoprotein.
- Decrease in the availability of ATP
- Pyridoxine and Pantothenic acid deficiency
Lipotropic factors
- Deficiency of lipotropic factors causes TAG to accumulate in liver.
- Choline, Betaine, methionine, Inositol, Folic acid, Vitamin B12, Glycine, Serine.