High Energy Compounds
- Substances which possess sufficient free energy to liberate at least 7 Cal/mol at pH 7.0
- Possess high energy bonds: acid anhydride bonds.
| Class | Examples of high-energy compounds |
| Pyrophosphates | ATP, pyrophosphate |
| Acyl Phosphates | 1,3 Biphosphoglycerate,
Carbamoyl phosphate |
| Enol Phosphate | Phosphoenol pyruvate |
| Thiol esters | Acetyl CoA, Acyl CoA |
| Guanidino phosphates | Phosphocreatine,
Phosphoarginine |
ATP: AMP ~ P ~ P
- Energy currency of the cell.
- Energy link between catabolism & anabolism.
- Donates high energy phosphates to low energy compounds, to make them energy rich.
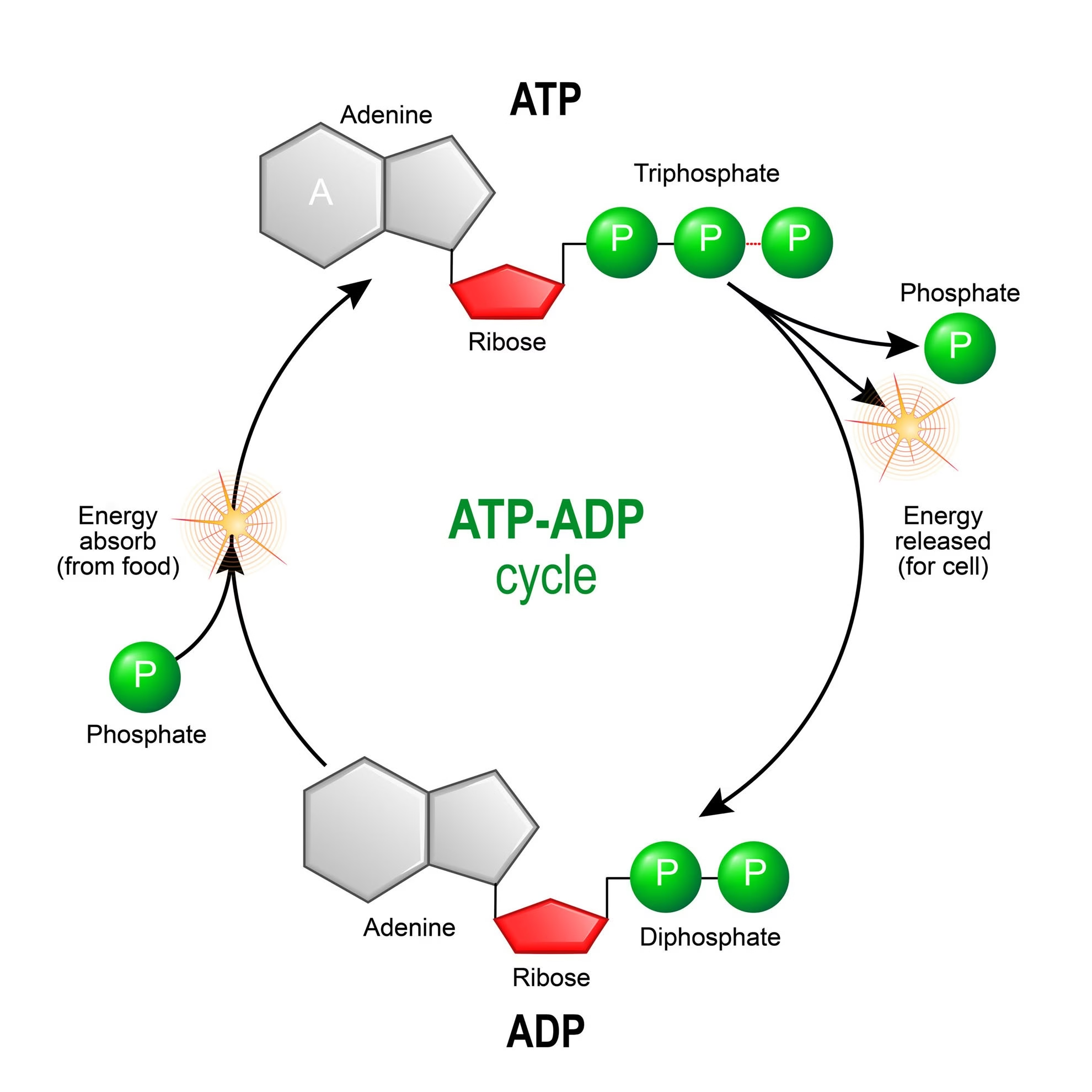
Fig: ATP-ADP Cycle: [ATP links the two major components of metabolism – catabolism (breakdown of biomolecules) and anabolism (synthesis of biomolecules)].
Enzymes involved in Biological Oxidation
The crucial enzymes of biological oxidation belong to the group oxidoreductases. They are classified into four categories.
- Oxidases
- Dehydrogenases
- Hydroperoxidases
- Oxygenases
Oxidases
Catalyse the elimination of hydrogen from substrates which is accepted by oxygen to form water.
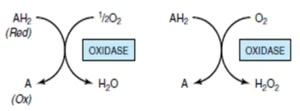
Cytochrome oxidase/ Cytochrome a,a3 :
- Hemoprotein, terminal component of ETC.
- Transfers electrons from complex III to final acceptor 02.
- Flavoprotein linked oxidases :
L amino acid oxidases (FMN), Xanthine oxidases (FAD)
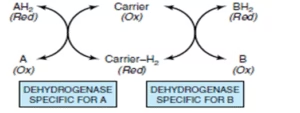
Dehydrogenase
These enzymes transfer hydrogen from one substrate to another in a coupled oxidation–reduction reaction.
Dehydrogenases cannot use Oxygen as a hydrogen acceptor.
- a) Cytochromes: b,c1, c of ETC
- b) Dehydrogenases dependent on niacin coenzymes:
- NAD+ dependent dehydrogenases
- NADP+ dependent dehydrogenases
- c) Dehydrogenases dependent on flavin coenzymes:
- FMN-dependent dehydrogenases
- FAD dependent dehydrogenases
Hydroperoxidases:
- Example: A. Peroxidases
- Prevents harmful effects of H2O2.

- Ex : Glutathione peroxidase (RBC)
- B. Catalases
- Catalyse H2O2 destruction & protects Hb.

- Catalase – hemoprotein, 4 heme groups.
Oxygenases:
Direct incorporation of O2 into substrate.
- Dioxygenase :
A + O2 → A02
Ex : Homogentisate oxidase.
- Monoxygenases : (mixed function oxygenase)
A-H + O2 + ZH2 → A-OH +H20 + Z
Ex : Cyt P 450 – detoxification of many drugs.
Biological Oxidation
The entire sequence of events involving oxidation of foodstuffs finally yielding energy is known as biological oxidation. Biological oxidation is the process in which substances (carbohydrate, Lipid, Amino acids) are oxidized in living organisms.
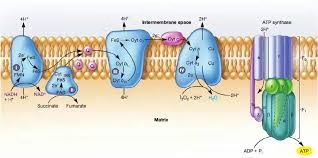
Sequence of electron carriers in inner mitochondrial membrane are organized into Electron Transport Chain ETC.
The cells consuming oxygen and producing carbon dioxide coupled with energy generation is the essence of respiration. This is the reason the Electron Transport Chain is also called as the Respiratory chain.
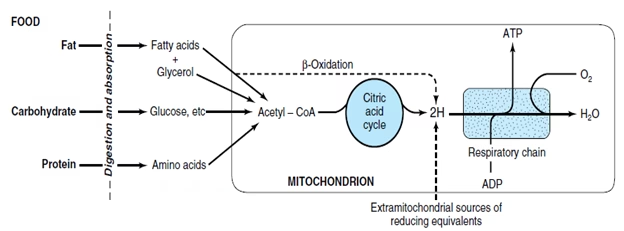
Fig: Overview of ETC
Electron Transport: Electrons carried by reduced coenzymes (NADH or FADH2) are passed sequentially through a chain of proteins and coenzymes (so called electron transport chain) to O2.
- Oxidation :- Loss of electrons.
- Reduction :- Gain of electrons.

Components of the respiratory chain (Electron Transport Chain):
- Nicotinamide nucleotides
- Flavin nucleotides/ Flavoproteins
- Iron–sulphur proteins
- Coenzyme Q
- Cytochromes
All the components are located on inner mitochondrial membrane.
- Respiratory or Enzyme complexes I,II, III, IV : Carriers of electrons.
- Complex V : ATP synthesis
- Mobile electron carriers: NADH, coenzyme Q, Cytochrome c, oxygen.
- Largest portion of O2 supplied to the body is utilized by mitochondria for ETC.
I. Nicotinamide nucleotides:
-
- NAD+, NADP+ are 2 coenzymes from niacin
- NAD+ actively involved in ETC
- AH2 + NAD → A + NADH + H+
- Substrates : Glyceraldehyde 3 phosphate, pyruvate, isocitrate, alpha KG , malate
- Dehydrogenation Of substrate: 1st step ETC.
- NADPH + H : Not suitable for ETC
II. Flavin Nucleotides/ Flavoproteins
-
- Components of complex I & II.
- NADH dehydrogenase (NADH-coenzyme Q reductase); FMN Prosthetic group.
- NADH + H+ + FMN → NAD + FMNH2
- Succinate dehydrogenase (Succinate- coenzyme Q reductase); FAD prosthetic group.
- Succinate + FAD → Fumarate + FADH2
III. Iron sulphur protein / Non heme iron proteins:
-
- Exist Fe3+ (ox) or Fe2+ (red) state.
- Found in complexes I, II, III.
- One FeS — Transfers electron from FMN to coQ.
- 2nd FeS — Transfer electron from Cyt b to cyt c1
IV. Coenzyme Q
-
- Ubiquinone: Lipophilic electron carrier.
- Quinone derivative with Isoprenoid side chain.
- In mammalian CoQ10.
Accepts electron from FMNH2 in ETC by NADH dehydrogenase or FADH2 outside ETC by succinate dehydrogenase.
V. Cytochromes
-
- Conjugate Protein: Heme group (Porphyrin + Fe)
- Iron of Heme: alternately oxidised (Fe3+) & reduced (Fe2+), which is essential for transport of electrons in ETC.
- Cytochrome are b, c1, c, a, a3.
- Electrons transported from ,
- CoQ ⇒ Cyt b ⇒ Cyt c1⇒ Cyt c ⇒ Cyt a ⇒ Cyt a3 ⇒H2O
- Cyt a & a3 called Cytochrome oxidase.
Route-map for electron flow
Complex I: NADH to Coenzyme Q
Complex II: Succinate to Coenzyme Q
Coenzyme Q
Complex III: Co Q to Cytochrome C
Cytochrome C
Complex IV: Cytochrome C to Oxygen
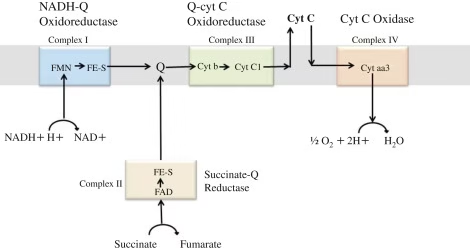
Fig: Organization of ETC
Inhibitors of ETC
Complex I: Rotenone, Amobarbital, Piercidin A
Complex II: Malonate, Carboxin, TTFA
Complex III: BAL, Antimycin A
Complex IV: H2S, Carbon Monoxide (CO), Cyanide, Hydrogen sulphide
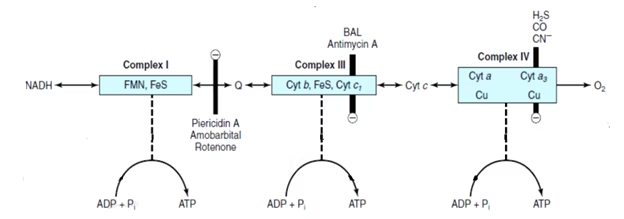
Fig: Inhibitors of ETC
Oxidative Phosphorylation
Definition: Electron transport through ETC is coupled with release of free energy. Coupling of electron transport (oxidation) and ATP synthesis (phosphorylation). It all happens at the inner mitochondrial membrane.
The synthesis of ATP from ADP & Pi in mitochondria by AYP synthase coupled with electron transport through respiratory chain is known as Oxidative phosphorylation.
Sites of oxidative Phosphorylation:
Flow of two electrons via complexes I,III, IV results in translocation of total 10 proteins out of the mitochondrial matrix. It is estimated that synthesis of one molecule of ATP requires involvement of four protons.
Complex I (NADH-Coenzyme Q Oxidoreductase): 4 protons: 1 ATP
Complex III (Coenzyme Q- Cytochrome C Oxidoreductase): 2 protons: 0.5 ATP
Complex IV (Cytochrome Oxidase): 4 Protons: 1 ATP
These coupling sites of oxidation-reduction are called as Redox Loops.
P: O ratio:
- The P: O ratio can be defined as the number of inorganic phosphate (Pi) molecules utilized for ATP production for every atom of oxygen consumed.
- Number of molecules of ATP formed per pair of electron passing through ETC.
- NADH oxidation: P:O ratio is 2.5
- NADH + H+ ½ O2 + 3 ADP + 3 Pi ⇒ NAD+ + 3ATP + 4 H2O.
- Oxidation of FADH2: P:O ratio is 1.5
- The oxidation of one molecule of NADH through ETC and oxidative phosphorylation yields about 2.5 ATP and the oxidation of one molecule of FADH2 yields 1.5 ATP. (Only two coupling sites for oxidation of FADH2, first site-complex I is bypassed and complex II does not pump protons)
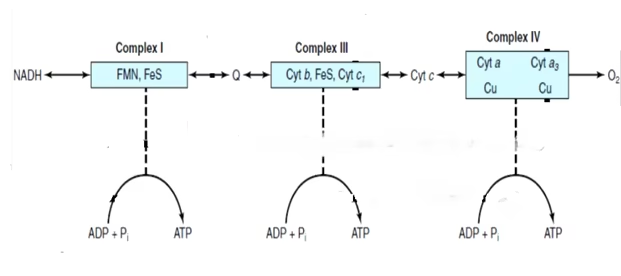
Fig: Sites of Oxidative Phosphorylation
Mechanism of Oxidative phosphorylation
Two hypotheses:
Chemical coupling hypothesis:
- Edward Slater states during electron transfer in ETC, a series of phosphorylated high energy intermediates produced which are utilized for ATP synthesis.
- Analogous to substrate level phosphorylation.
- Lacks experimental evidence.
Chemiosmotic hypothesis
- Peter Mitchell (1961): widely accepted.
- Transport of electrons through ETC is coupled with translocation of protons (H+) across inner mitochondrial membrane from matrix to intermembrane space.
- Electrochemical or Proton gradient.
- Protons re-enter the matrix leading to ATP synthesis.
The essence of chemiosmotic theory is, the free energy generated from the electron flow through ETC is conserved by pumping protons out of the matrix to create an electrochemical proton gradient across the inner mitochondrial membrane. The electrochemical potential of this proton gradient is used to synthesise ATP. The electrochemical gradient or proton gradient thus developed, acts as the power that drives the mechanism operating the synthesis of ATP.
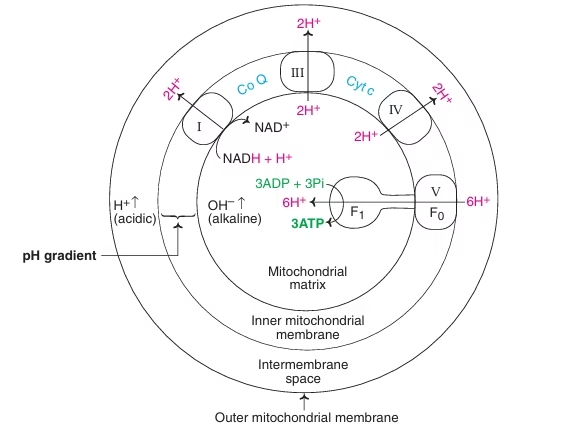 Fig: Chemiosmotic theory of oxidative phosphorylation
Fig: Chemiosmotic theory of oxidative phosphorylation
Rotary motor model
- Paul Boyer (1964).
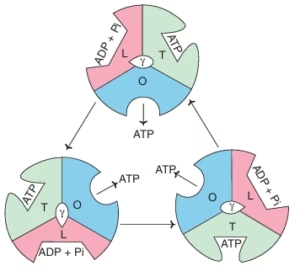 Fig; Structure of ATP synthase
Fig; Structure of ATP synthase - Engine driving/ binding change model.
- ATP synthase: F0F1 complex.
- World’s smallest molecular motor.
- Three β subunits adopt different conformations:
- Loose (L), Tight (T), Open (O).
Inhibitors of Oxidative Phosphorylation
- Valinomycin & Nigercin: K+ inophores — disturb proton gradient.
- Oligomycin: binds with enzyme ATP synthase. Inhibits the translocation of protons.
- Actractyloside: Plant toxin.
- Inhibits adenosine nucleotide carrier system.
- Blocks the adequate supply of ADP.
Uncouplers of Oxidative Phosphorylation
- ETC is tightly coupled with oxidative phosphrylation.
- Uncouplers delink ETC from oxidative phosphorylation.
- Increases permeability of inner mitochondrial membrane to protons & hence ATP synthesis does not occur.
- Energy liberated via transport of electrons is dissipated as heat.
- Uncouplers allow oxidation of substrate without ATP formation.
Ex: 2,4 dinitrophenol
- -Dinitrocresol – pentachlorophenol
- – FCCP – Aspirin in high doses.
Physiological uncouplers:
- Thermogenin – Thyroxine
- Long chain fatty acids. – Unconjugated bilirubin.
Brown adipose tissue:
- Colour from high density of mitochondria in the adipose cells.
- Their mitochondria contain
- Increases permeability of inner mitochondrial membrane to protons & dissipates proton gradient, hence ATP synthesis does not occur.
- Phosphorylation uncoupled from oxidation leading to release of energy as heat.
Disorders associated with ETC and Oxidative phosphorylation:
- Alzheimer’s and Parkinson’s diseases
- Leber’s hereditary optic neuropathy
- MELAS (Mitochondrial Encephalopathy, Lactic Acidosis and Stroke)
Substrate Level Phosphorylation
- Energy from high energy compounds is directly transferred to nucleoside di-phosphate to form tri-phosphate compound without help of ETC.
- ATP directly synthesized.
- Glycolysis intermediates: 1,3 bisphosphoglycerate, Phosphoenolpyruvate
- TCA intermediates : Succinyl CoA