Introduction
Acid‑base balance refers to the processes by which the body maintains the hydrogen ion concentration ([H⁺]) in body fluids—especially in the blood—within a narrow, physiologically acceptable range. This is critical because many biochemical reactions, enzyme activities, and cellular functions are exquisitely sensitive to pH (which is a measure of [H⁺]).
In humans, the normal arterial blood pH is about 7.35 to 7.45 (slightly alkaline). Deviations outside this range (even modest ones) can impair cellular function, disrupt metabolism, or affect cardiovascular and respiratory systems.
Maintenance of Blood pH
There are three lines of defence operative in maintaining the blood pH.
- Buffer systems
- Respiratory mechanism
- Renal regulation
Buffer systems
- Buffers are the solutions which resist change in pH by the addition of small amounts of acids or bases. Any substance that can reversibly bind hydrogen ions can be labelled as a buffer.
- Buffers can neither remove H+ ions from the body nor add them to it. They can only keep H+ ions in a temporarily suspended form. The H+ ions have to be ultimately eliminated by the kidneys.
- Take up H+ or release H+ as conditions change.
- Buffer pairs – weak acid and its salt with strong base.
- Results in a much smaller pH change.
- Buffering capacity: absolute conc. of salt & acid.
- Buffer acts quickly but not permanently.
- There are three major buffer systems operative in the human body.
-
- Bicarbonate buffer
- Phosphate buffer
- Protein buffer
Bicarbonate buffer:
- Bicarbonate and carbonic acid pair (NaHCO3– H2CO3–) is the predominant buffer system in the extracellular fluid.
- Predominant buffer system of ECF.
- Sodium Bicarbonate (NaHCO3) and carbonic acid (H2CO3).
- The concentration of the bicarbonate ion is not directly measured; it is calculated from the Henderson–Hasselbalch equation.
- The Henderson–Hasselbalch equation for the bicarbonate buffer pair is
- Maintain a 20:1 ratio: HCO3– : H2CO3
- Alkali reserve: Concentration of bicarbonate is almost 20 times more than that of carbonic acid in the blood. This is referred to as the alkali reserve and is essential to meet the challenge of buffering the H+ ions generated from the huge body acid–load.
Phosphate buffer:
- Major intracellular buffer
- NaH2PO4 – Na2HPO4.
- Ratio of base to acid is 4.
Protein Buffer:
- Includes plasma protein & hemoglobin, work in blood and ISF
- Imidazole group of Histidine (6.8)
- 2% of total buffering capacity of the plasma
Respiratory Mechanisms
- Exhalation of carbon dioxide
- Powerful, but only works with volatile acids.
- Rapid & short term (several minutes to hours).
- Doesn’t affect fixed acids like lactic acid
- CO2 + H20 ↔ H2CO3 ↔ H+ + HCO3–
- Body pH can be adjusted by changing rate and depth of breathing.
- Rate of respiration: Respiratory centre in medulla.
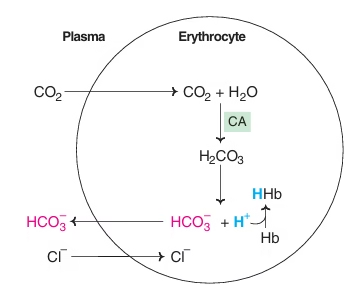
Fig: Generation of bicarbonate by erythrocytes
Chloride shift:
As concentration of HCO3- increases in RBC, it diffuses into plasma along concentration gradient, in exchange for Cl-, to maintain electrical neutrality- Chloride shift
Isohydric Transport:
- At tissue level, Hb binds H+ ions and helps to transport CO2 as HCO3- with a minimum change in pH.
- In the lungs, Hb combines with O2, H+ ions are removed which combine with HCO3- to form H2CO3. (CO2 exhaled.)
Renal Mechanism
- Can eliminate large amounts of acid
- Most effective regulator of pH
- Permanent but takes several hours to days.
- pH of urine is acidic (6.0).
- Maintains alkali reserve.
- Excrete or reabsorb acidic or basic substances.
- If kidneys fail, pH balance fails
The basic mechanisms of renal regulation of pH include:
- Reabsorption of bicarbonate
- Addition of new bicarbonate to the plasma
- Excretion of ammonium ions (Half to two-thirds of body acid load is eliminated)
- Excretion of free H+
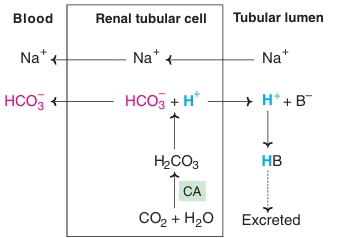
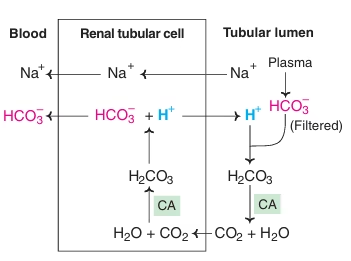
Fig: Reabsorption of bicarbonate
- Reabsorption of bicarbonate:
In normal conditions, the entire HCO3- filtered by the glomeruli is returned to the body by renal tubular cells. Hence normal urine is bicarbonate free. The enzyme carbonic anhydrase plays the central role in these mechanisms. If HCO3- in glomerular filtrate is not reabsorbed, it is excreted in the urine depleting the body’s alkali reserve causing acidosis.
Fig: Addition of new bicarbonate to the plasma
- Addition of new bicarbonate to the plasma: (Excretion of H+ ions as titratable acid)
H+ ions combine with a filtered non-bicarbonate buffer in tubular lumen (HPO4-) forming H2PO4 ion and are excreted in the urine.
There is net loss of H+ ions in urine as a component of non-bicarbonate buffer. The HCO3- formed in the cells is obtained from cellular CO2 and hence it is considered as a net gain of bicarbonate.
Fig: Excretion of ammonium ions
- Excretion of ammonium ions:
NH3, formed in renal tubular cell from glutamine by the enzyme glutaminase, diffuses into tubular lumen where it combines with H+ to form NH4+. Bulk of body acid load is eliminated in the form of NH4+.
- Excretion of free H+ ions:
Quantitatively loss of H+ ion in its free form is very negligible.
- The two most important urinary buffers other than bicarbonate are phosphate and ammonia.
- They not only buffer H+ ions but are also involved in HCO3- generation.
- Amount of new HCO3- added to the plasma by the kidneys is proportional to the amount of H+ ions excreted in urine.
Rates of correction:
- Buffers function almost instantaneously
- Respiratory mechanisms take several minutes to hours
- Renal mechanisms may take several hours to days
pH, acid, base, buffers
- Acids: substances that can donate protons [H+] in solution
- Bases: The substances that can accept protons [H+] in solution.
- pH: Negative logarithm of [H+] ion concentration.
- Buffer: A mixture of a weak acid and a salt of its conjugate base that resist changes in pH when a strong acid or base is added to the solution.
Acids and bases can be:
Strong – dissociate completely in solution Example: HCl, NaOH
Weak – dissociate only partially in solution Example: Lactic acid, carbonic acid
pH:
- Power of H+ ion concentration
- pH = – log [H+]
- Range is from 0 – 14 (1M to 10-14 M of H+ ion conc)
- If [H+] is high, the solution is acidic; pH < 7
- If [H+] is low, the solution is basic or alkaline ; pH > 7
The Body and pH:
- Homeostasis of pH is tightly controlled
- Extracellular fluid = 7.4
- Blood = 7.35 – 7.45
- < 6.8 or > 8.0 death occurs
- Acidosis (acidemia) below 7.35
- Alkalosis (alkalemia) above 7.45
Fig: The body and pH
Small changes in blood pH can produce major disturbances:
- Most enzymes function only with narrow pH ranges
- Acid-base balance can also affect electrolytes (Na+, K+, Cl–)
- Can also affect hormones & distorts protein structure.
Anion Gap
- Total concentrations of cations & anions are equal in the body fluids.
- Na+ & K+: 95% of plasma cations.
- Cl- & HCO3- : 80% of plasma anions.
- Unmeasured anions in the plasma A-
- Proteins, phosphate, sulfate, urate.
- It can be calculated as follows:
- Anion gap (A–) = ([Na+] + [K+]) – ([Cl–] + [HCO3–])
= (136 + 4)– (100 + 25)
A– = 15 mEq/L
- The anion gap in normal health is around 15 mEq/l (12-18 mEq/l ).
- The alterations in the anion gap are useful in the clinical assessment of acid–base disorders.
Acid-Base Disorders:
- pH< 7.35 acidosis
- pH > 7.45 alkalosis
- Metabolic Acidosis: due to decrease in bicarbonate (HCO3-)
- Metabolic Alkalosis: due to increase in bicarbonate (HCO3-)
- Respiratory Acidosis: due to increase in carbonic acid (H2CO3)
- Respiratory Alkalosis: due to decrease in carbonic acid (H2CO3)
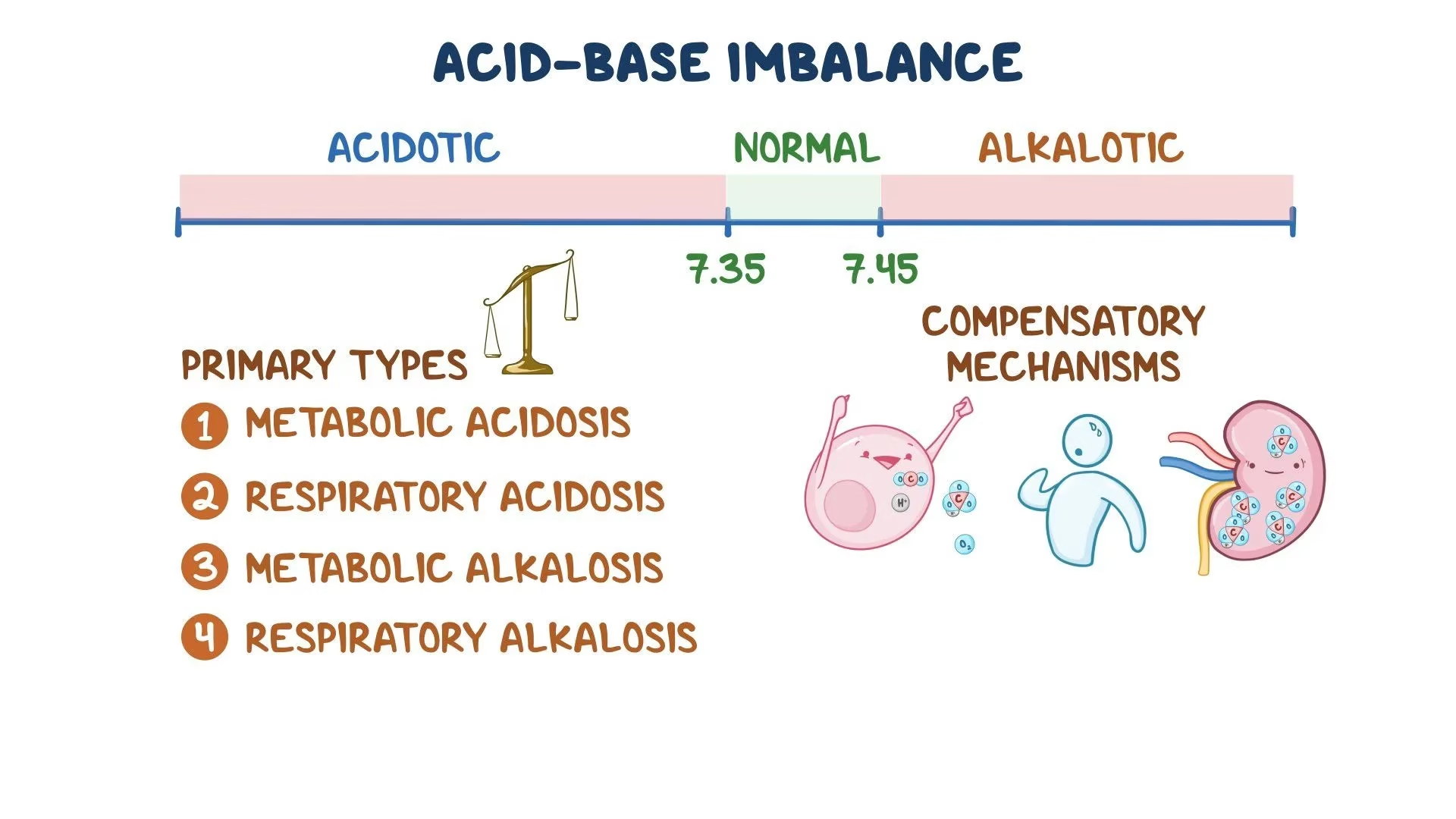
Fig: Acid-base imbalances
Acidosis:
- Principal effect of acidosis:
- Depression of the CNS through decrease in synaptic transmission.
- Deranged CNS function: the greatest threat
- Generalized weakness
- Severe acidosis causes: Disorientation, Coma, Death
Alkalosis:
- Alkalosis causes over excitability of the central and peripheral nervous systems.
- Numbness
- Lightheadedness
- It can cause :
- Nervousness
- Muscle spasms or tetany
- Convulsions
- Loss of consciousness
- Death
- The body response to acid-base imbalance is called compensation
- May be complete if brought back within normal limits
- Partial compensation if range is still outside norms.
- If underlying problem is metabolic (due to changes in HCO3-), hyperventilation or hypoventilation can help: respiratory compensation (regulates the H2CO3).
- If underlying problem is respiratory, (due to changes in H2CO3), renal mechanisms can bring about metabolic compensation (regulates the HCO3-).
Causes of Acid-base disorders: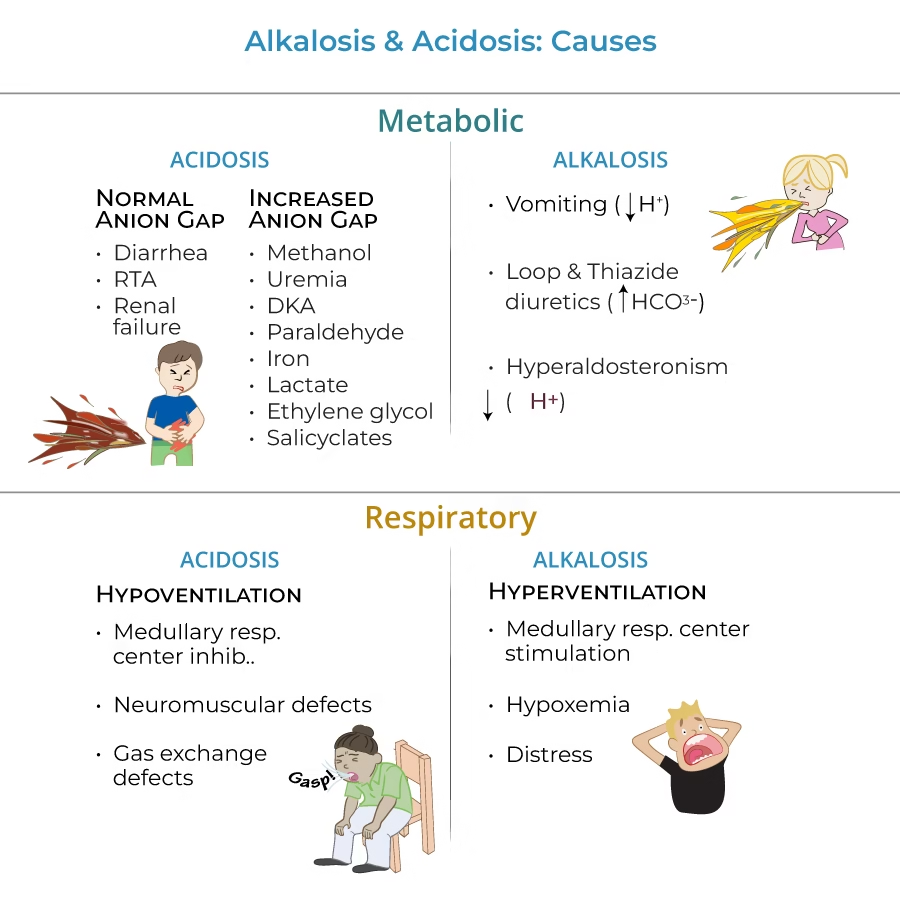
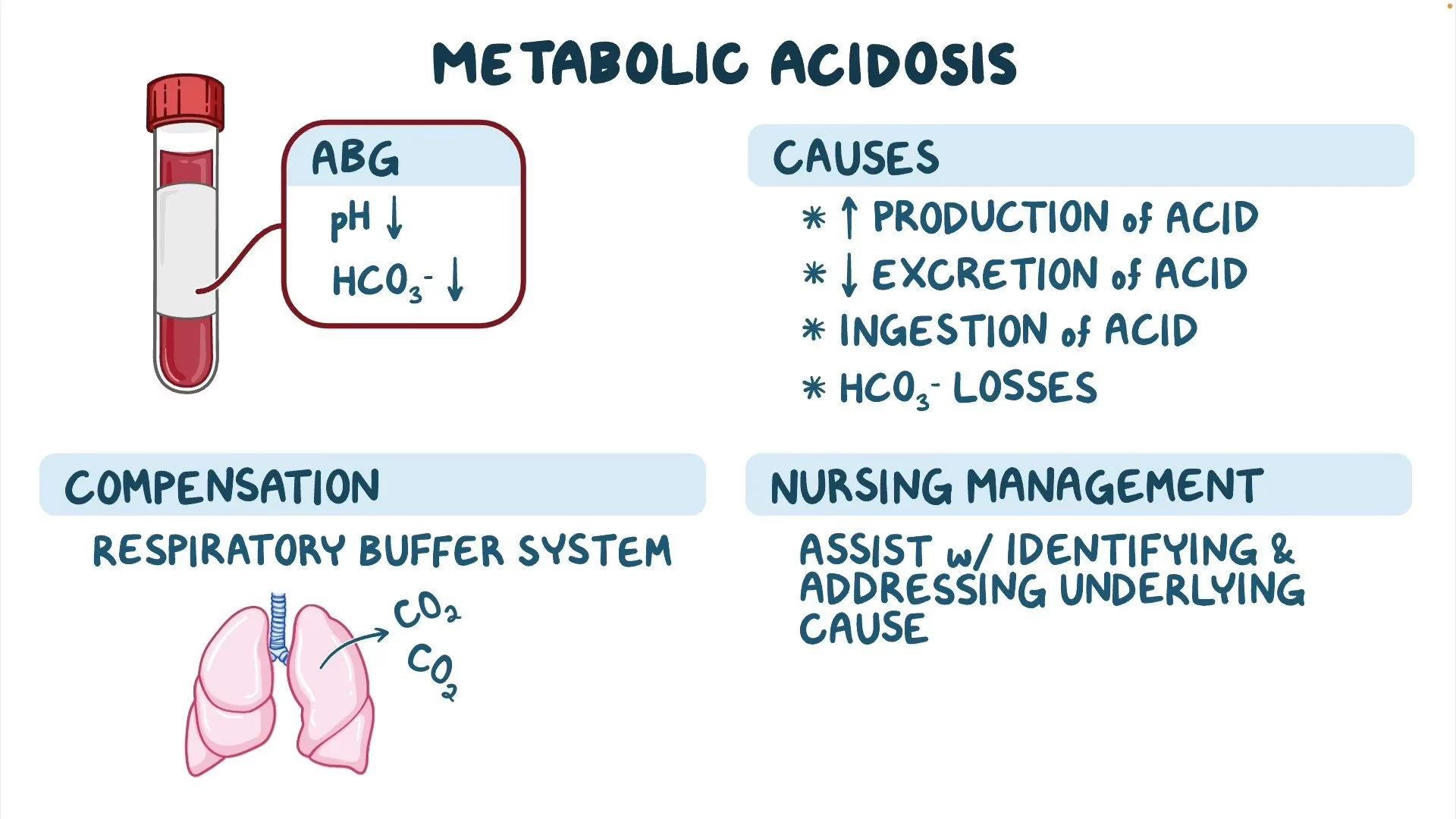
Metabolic Acidosis
The primary abnormalities in metabolic acidosis are:
- Increased production or decreased excretion of H+ ions or both at once.
- Loss of HCO3– from the body
Causes of metabolic acidosis
- Severe uncontrolled diabetes mellitus
- Diabetic ketoacidosis (DKA).
- Starvation ketosis,
- Lactic acidosis
- Renal failure
Compensation:
- Respiratory- Hyperventilation (elimination of CO2): H2CO3 ↓
- Renal excretion of H+ ions.
Management: Metabolic acidosis can be effectively managed by identifying and treating the underlying cause. This includes treatment of diabetes, control of diarrhoea, and correction of shock and so on.
- Oral or IV NaHCO
- IV lactate solution (converted to HCO3- in liver).
High Anion Gap Metabolic Acidosis:
- Lactic acidosis
- Keto acidosis (Diabetes, Alcohol, Starvation)
- Toxins (Ethylene Glycol, Methanol, Salicylates)
- Renal Failure
Metabolic Alkalosis
The primary abnormality in metabolic alkalosis is an increase in plasma HCO3– concentration with a consequent reduction in H+ ion.
Causes
- Sustained vomiting
- Bicarbonate administration for prolonged periods as a means of treating indigestion and overuse of antacids for treatment of dyspepsia result in metabolic alkalosis.
- Overdose of diuretic drugs
- Mineralocorticoids excess (Cushing’s syndrome, Conn’s syndrome), alkalosis occurs due to increased H+ ion excretion in the urine.
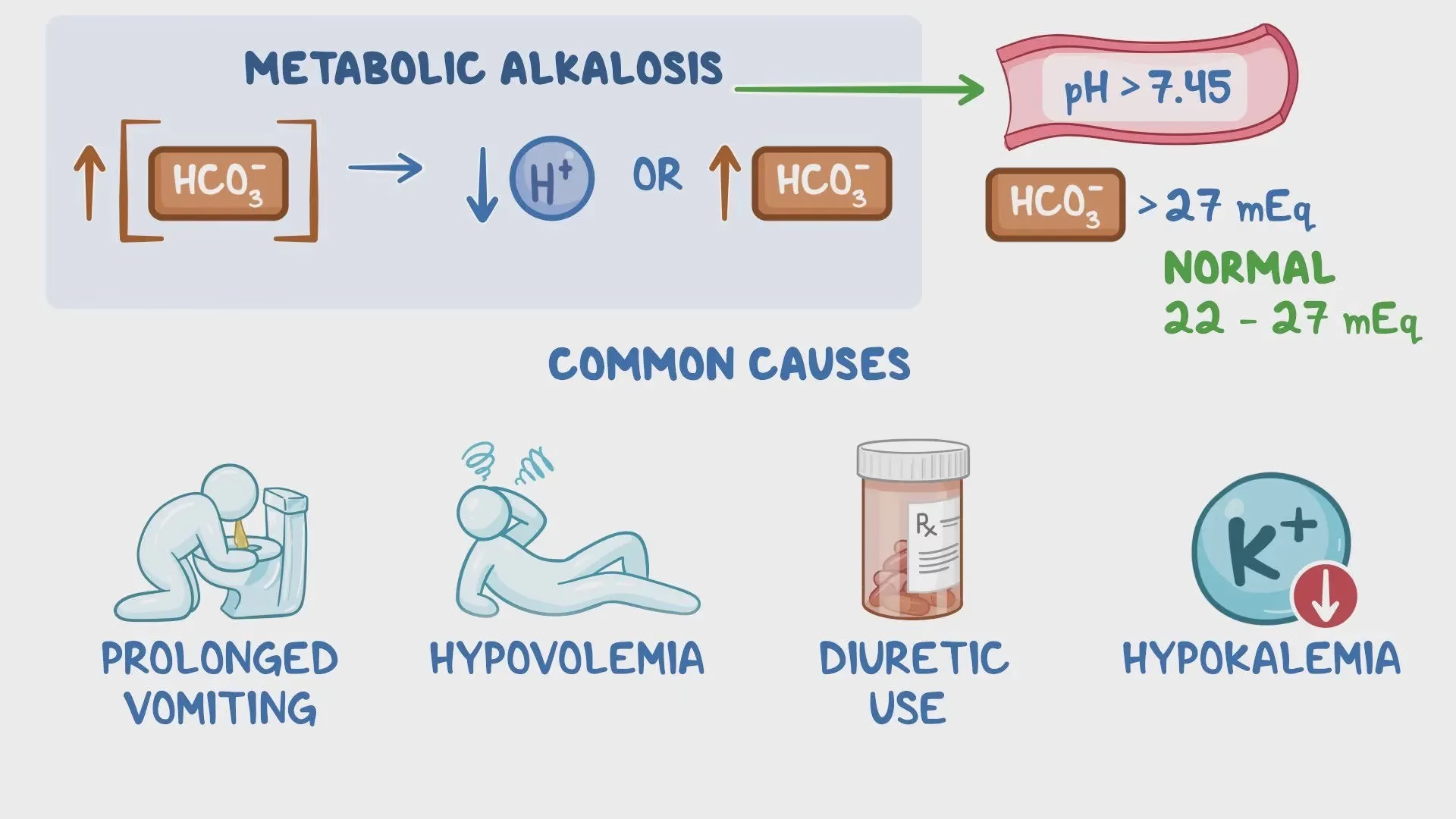
Compensation:
- Respiratory compensation– hypoventilation to retain CO2 (hence H2CO3 ↑).
- Renal mechanism excretes more HCO3– and retains H+.
Principles of management:
- Correcting the underlying cause, adequate intravenous fluid administration and replacement of K+ correcting hypokalemia.
- IV chloride containing solution. (HCO3– ions are replaced by Cl- ions).
Respiratory Acidosis
The primary abnormality in respiratory acidosis is retention of CO2 (H2CO3) due to impaired alveolar ventilation resulting in a rise in pCO2.
Causes:
- Chronic
- Depression of respiratory center – drugs opiates or head trauma
- Paralysis of respiratory or chest muscles
- Emphysema, asthma, pneumonia, COPD
- Causes: Acute
- ARDS, pneumothorax
Compensation:
- Kidneys: excrete hydrogen ion & titratable acidity.
- Retain bicarbonate ion and ads up to the alkali reserve of the body.
Principles of management:
- Restore ventilation and lower the pCO2
- IV lactate solution (converted to HCO3– in liver).
- Treat underlying dysfunction or disease.
Respiratory Alkalosis
The primary abnormality in respiratory alkalosis is a decrease in H2CO3 concentration. A fall in pCO2 reduces the ratio of carbonic acid to bicarbonate resulting in acidosis.
Most common acid-base imbalance.
Causes:
- Primary cause is hyperventilation.
- Conditions that stimulate respiratory center:
- Hyperventilation resulting from hysteria and anxiety states cause respiratory alkalosis.
- Raised intracranial pressure and brain stem lesions cause hyperventilation by stimulating the respiratory centre. Ingestion of certain drugs (Salicylate) also stimulates the brain stem respiratory centre.
- Hypoxia occurring in high altitudes, severe anaemic conditions and pulmonary disease.
Compensation:
- Kidneys: conserve hydrogen ion.
- Excrete bicarbonate ion.
Principles of management:
- Treat underlying cause
- Patient is subjected to re-breathing into a closed bag to allow CO2 levels to rise
- IV Chloride containing solution: (Cl– ions replace lost bicarbonate ions)