Integration and Starvation
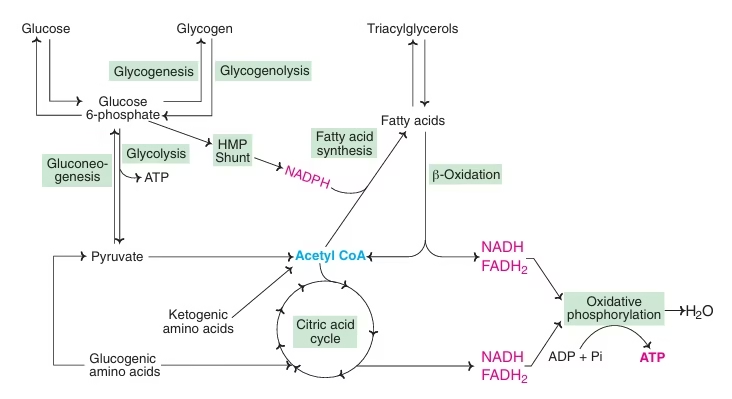 Fig: Metabolic interrelationship of major pathways of energy metabolism
Fig: Metabolic interrelationship of major pathways of energy metabolism
Metabolic integration
Phases of catabolism [All the major biomolecules – carbohydrates, lipids and proteins undergo catabolism to finally yield acetyl CoA which is further oxidised in the citric acid (Krebs) cycle releasing carbon dioxide and reducing equivalents (NADH + H+ and FADH2). Reducing equivalents then enter into the respiratory chain, ultimately producing ATP through the process of oxidative phosphorylation].
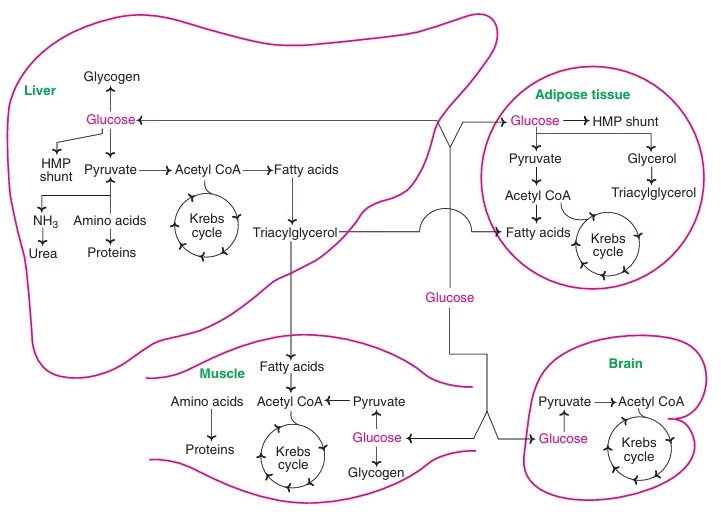 Fig: The inter-relationships between the metabolic pathways of carbohydrates, lipids and proteins.
Fig: The inter-relationships between the metabolic pathways of carbohydrates, lipids and proteins.
Starve-Feed Cycle
- Well-fed or Post absorptive state -> after a meal
- Early fasting state -> during the night
- Re fed state -> after breakfast
Studying the metabolic changes after an evening meal, fasting at night and the refed state after breakfast is known as the fast–feed cycle or starve–feed cycle.
The fed state:
- The biochemical changes following the consumption of food constitute the well-fed or post-prandial state.
- The carbohydrate content of the diet after digestion is mostly absorbed as glucose and enters the blood. The rise in blood glucose levels induces the beta cells of the islets of Langerhans in the pancreas to secrete
- Within the cells, the glucose is converted to pyruvate or lactate by glycolysis or it is used in the HMP shunt for the formation of NADPH and pentose sugars.
- Availability of a plentiful supply of glucose in the well-fed state coupled with the high insulin levels result in the storage of glucose as glycogen (glycogenesis) in the liver and muscle.
- Dietary proteins are digested and absorbed as amino acids in the intestine. Very small amounts are used by the intestinal cells and the rest is transported through portal blood to the liver and all other tissues where they are primarily used for protein synthesis.
- The dietary lipids are packaged into chylomicrons in the intestinal epithelial cells and are transported to the blood through the lymphatic system.
Fig: Metabolism in fed state
The fasting state:
- The fasting state constitutes the period after an evening meal, extending into the fasting state during the night until breakfast the next morning.
- The glucose levels in the blood start dropping after a few hours during the post absorptive period. The fall in blood glucose decreases insulin secretion and stimulates glucagon secretion. Glucagon stimulates glycogen breakdown and inhibits glycogen synthesis
- As a result, Glycogenolysis in the liver contributes to blood glucose in the early fasting state.
- In addition, glucagon also inhibits glycolysis and fatty acid synthesis
- The liver glycogen levels are depleted by late night and the early hours of the morning (8–10 hours after supper), and the body switches over to hepatic gluconeogenesis to maintain the blood glucose levels.
- Since the fatty acids cannot be used for the synthesis of glucose, the net glucose synthesis by gluconeogenesis derives its carbon source from glycerol and muscle proteins.
- A substantial amount of acetyl CoA produced by fatty acid oxidation is used for the formation of ketone bodies
Starvation:
- Deprivation of food and thereby deprivation of exogenous supply of calories to meet the energy demands of the body for basal metabolism and other activities.
- May be due to food scarcity or the desire to rapidly lose weight or certain clinical conditions (surgery, burns etc.)
- When the fasting state is prolonged, it results in starvation. During starvation, the entire body metabolism is reorganised for the survival of the organism.
- Starvation is a metabolic stress which imposes certain metabolic impulsions on the organism.
- Biochemical changes that occur during starvation are such that an adequate supply of fuel molecules is maintained to various tissues to meet the energy demands.
Metabolic profile- Liver
WELL FED ABSORPTIVE STATE:
- Glucolysis, Glycogenesis , HMP shunt ↑
- Gluconeogenesis ↓
- Lipogenesis ↑
- Amino Acids → Proteins (↑ Protein synthesis)
Starvation:
- Decrease in Insulin/ Glucagon ratio.
- Glycogenolysis ↑
- Gluconeogenesis↑
- Fatty Acids oxidation ↑
- TCA cycle cannot utilize excess acetyl CoA.
- Ketogenesis↑
Metabolic Profile: Adipose tissue
Well fed absorptive state:
- Uptake of glucose (↑ Glycolysis & HMP shunt)
- Synthesis of Fatty Acids & Triacylglycerol (↑ Lipogenesis)
- Degradation of Triacylglycerol decreased (↓ Lipolysis)
Starvation:
- Glucose uptake & metabolism ↓
- ↓ Lipogenesis & ↑ Lipolysis : Free Fatty Acids synthesis ↑
Metabolic profile – Muscle:
Well fed absorptive state:
- Glucose & Fatty Acids utilized ( ↑ Glycolysis, Glycogenesis)
- Amino Acids → Proteins (↑ Protein synthesis)
Early starvation:
- Glucose uptake depressed.
- Both Fatty Acids & Ketone Bodies utilized.
- Proteins → Amino Acids (Utilized in Gluconeogenesis)
Prolonged starvation:
- Exclusive Ketone Bodies.
- Protein breakdown ↓ decreased.
Metabolic profile- Brain:
- Glucose is the only fuel for human brain -> consumes 120g/day -> 60-70 % of utilization of glucose.
- Free Fatty Acids cannot cross Blood-Brain-Barrier.
- In starvation ->Beyond 3 weeks Ketone bodies can replace glucose.
Metabolic profile – Kidney
- During starvation, the kidney becomes an important site of gluconeogenesis and may contribute as much as half of the blood glucose.
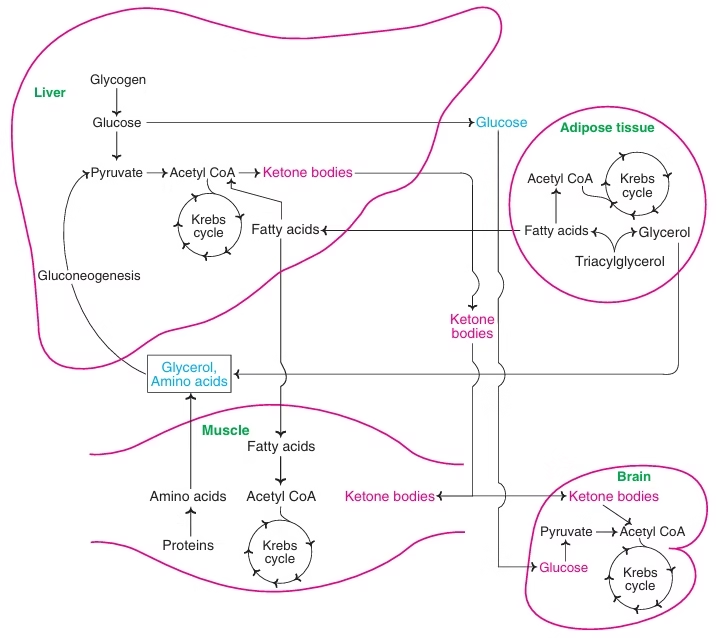 Fig: Metabolism in the fasting state
Fig: Metabolism in the fasting state