Chemistry of Nucleotides & Nucleic Acids
There are two types of nucleic acids: deoxyribonucleic acids (DNA) and ribonucleic acids (RNA). Primarily, nucleic acids serve as repositories and transmitters of genetic information. DNA was discovered in 1869 by Johann Friedrich Miescher, a Swiss Researcher. DNA contains genetic information was discovered by Avery, Macleod and McCarry in 1944.
Nucleotide:
Nucleotide, the basic unit of nucleic acids is composed of three components.
- Sugars in nucleic acids
- Phosphate group in nucleic acids
- Bases present in nucleic acids
- Purines
- Pyrimidines
Nomenclature of nucleotides:
Nucleoside = nucleobase + pentose sugar
Nucleotide = nucleoside + phosphate
Image: Nomenclature of bases, nucleotides and nucleotides
Functions of nucleotides
- Nucleotides are the building blocks of nucleic acids (DNA and RNA).
- ATP is the universal energy currency of living systems.
- Cyclic nucleotides such as cAMP and cGMP act as ‘second messengers’
- Nucleotides are the structural components of a number of coenzymes
- They act as the carriers of certain metabolic intermediates of carbohydrates, (UDP-glucose), lipids (CDP-acyl glycerol) and proteins (S-adenosyl methionine-SAM).
- UDP-Gal, UDP-Glu, GDP-Man, CMP-NeuAc, GDPFuc, UDP-Xyl, UDP-Gal Nac and UDP-Glu Nac – These eight molecules are known as ‘nucleotide-linked sugars’ and are important constituents of glycoproteins
Synthetic nucleotides:
Therapeutic applications
- Synthetic nucleosides, cytarabine and vidarabine in which ribose is replaced by arabinose are used in chemotherapy to treat cancers.
- Allopurinol is used in the treatment of hyperuricemia and gout.
- Synthetic analogues such as 6-mercaptopurine, 5-fluorouracil, 5-iodouracil, 3-deoxyuridine, 5 or 6-azauridine, 5- or 6-azacytidine, 8-azaguanine, 6-thioguanine are widely used by oncologists. They are incorporated into DNA just before cell division, thus blocking cell proliferation.
- Drugs like zidovudine which are used in the treatment of AIDS are synthetic nucleotide analogues with alterations in the sugar structure.
Nucleic Acids
Nucleic acids are polymers of nucleotides (polynucleotides) held by 3’ and 5’ phosphate bridges.
Nucleotides are made up of nitrogenous base, pentose sugar & phosphate.
Functions of Nucleic Acids:
- DNA is the chemical basis of heredity and may be regarded as reserve bank of genetic information.
- DNA is exclusively responsible for maintaining the identity of different species of organisms over millions of years.
- Every aspect of cellular function is under control of DNA.
- DNA is organized into genes- the fundamental unit of genetic information.
Structure of DNA
The double helical structure of DNA was first proposed by James Watson and Francis Crick in 1953.
Watson–Crick model of DNA double helix:
- A DNA molecule consists of two chains of deoxyribonucleotides coiled around each other in the form of a double helix. Two helical DNA chains wind around each other on the same axis to form a right handed double helix.
- The two chains are antiparallel – one strand runs in the 5′ to 3′ direction, while the opposite is in the 3′ to 5′ direction. The terminal residue whose C5′ is not linked to another nucleotide is called the 5′ end, and the terminal residue whose C3′ is not linked to another nucleotide is called the 3′ end.
- The two strands are held together by hydrogen bonds between a purine base on one strand and a pyrimidine base on the opposite strand. The ring structure of each base occurs in a flat plane perpendicular to the sugar–phosphate backbone, resembling the steps on a spiral staircase. The base pairing maintains a constant distance between the sugar–phosphate backbones of the two strands as they twist around each other.
- The hydrogen bonds are formed between a purine base and a pyrimidine base only. This specificity is imposed on the base pairings by the fixed location of the hydrogen bonds. If two purines face each other, they would not fit into the space available and two pyrimidines would be too far apart to form hydrogen bonds. Each nucleotide base of one strand is paired in the same plane with a base of the opposite strand.
- Three hydrogen bonds are formed between the purine, guanine and the pyrimidine, cytosine (G–C or C–G pairing), while only two hydrogen bonds can be formed between the purine, adenine and the pyrimidine, thymine (A–T or T–A pairing). As a result, G always pairs with C, and A with T. This is known as complementary base pairing. This specificity has tremendous significance in DNA self replication and transcription.
- It is more difficult to separate the paired DNA strands rich in G–C pairing because GºC (three double bonds) pairing is stronger than A=T (two double bonds) pairing.
- The deoxyribose and phosphate groups (sugar–phosphate backbone) are hydrophilic in nature and hence are on the outside of the double helix orienting towards the surrounding water molecules, while the hydrophobic bases are stacked inside.
- The coiling of two strands creates a major groove and a minor groove on the surface of the duplex. Proteins interact with DNA at these grooves without disrupting the double helix.
- Geometry of the DNA duplex: The width of a double helix is 20 Å. Each turn of the helix is 34 Å with 10 pairs of nucleotides, each pair is placed at a distance of 3.4 Å.
- Because of their length, DNA chains are described in terms of base pairs (bp) or thousands of base pairs (kilobase pairs or kb), e.g. [5 kb (virus), 2,50,000 kb (humans)].
- The two antiparallel strands of DNA are not identical in either base sequence or composition, but are complementary to each other. Wherever adenine occurs in one chain, thymine is present in the other and vice versa; similarly, wherever guanine occurs in one strand, cytosine is found in the other and vice versa.
- The DNA duplex is stabilised by two forces; hydrogen bonding between complementary base pairs and nonspecific base-stacking interactions.
- The strand that is transcribed or copied into the RNA molecule is referred to as the template strand or anti sense strand. The other strand is the sense or coding strand of that gene. However it is noteworthy that each DNA strand can act as a template for the synthesis of its complementary strand and hence hereditary information is encoded in the sequence of bases on either strand.
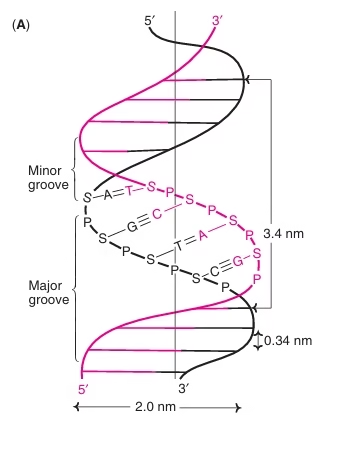 Fig: Watson–Crick model of DNA double helix
Fig: Watson–Crick model of DNA double helix
Chargaff’s rules:
- The base composition of DNA varies in different species.
- DNA from different tissues of the same species have the same base composition.
- The base composition of DNA in a species does not undergo any change with the organism’s age, nutritional status or changing environment.
- In all species, the number of adenine is equal to the number of thymine residues (A=T) and the number of guanine is equal to the number of cytosine residues (G º C). Thus, the sum of the purine residues equals the sum of the pyrimidine residues; that is, A+G = T + C
DIFFERENT TYPES (FORMS) OF DNA:
- B-DNA
- A-DNA
- Z-DNA
Unusual structures in DNA
- Hoogsteen pairing
- Triplex DNAs
- Tetraplex
- Palindromes
Denaturation of DNA
Disruption of hydrogen bonds ( by change in pH or increase in temperature) results in separation of polynucleotide strands. This phenomenon of loss of helical structure of DNA is known as denaturation. The phosphodiester bonds are not broken by denaturation.
Melting Temperature (Tm): is defined as the temperature at which half of the helical structure of DNA is lost.
Mitochondrial DNA:
- Apart from the nucleus, eukaryotic cells also have other organelles such as mitochondria and chloroplasts, which contain DNA. Mitochondrial DNA is capable of encoding certain proteins and RNA in mitochondria. The synthesis of about 13 proteins of the respiratory chain, are encoded by mtDNA.
- mtDNA is inherited only from the mother.
- Leber’s Hereditary optic neuropathy: due to mutations in mt DNA.
Centromeres and telomeres
- The nucleoproteins which link the chromosome to the mitotic spindle during cell division are anchored to a specific region on the DNA known as the
- This ensures an equal distribution of chromosome sets to daughter cells.
- The guanine-rich sequences at the ends of eukaryotic chromosomes are known as
- Telomeric DNA is synthesised and maintained by an enzyme known as The somatic cells of multicellular organisms lack telomerase activity (however their germ cells have active telomerase function). The loss of telomerase activity allows the gradual shortening of chromosomes with each cycle of DNA replication and cell division until they reach senescence (a stage at which there will be no more division). Perhaps this is the basis of ageing.
Nucleases:
Nucleases are enzymes that are capable of degrading nucleic acids.
- endonucleases
- exonucleases
- restriction endonucleases
Types of RNA:
- Messenger RNA (m-RNA)
Messenger RNA is located in the cytoplasm and transfers genetic information from the DNA to the protein-synthesising machinery on ribosomes.
- Transfer RNA (t-RNA)
It also occurs in cytoplasm, it translates the information carried by m-RNA into a specific sequence of amino acids that are incorporated into protein.
- Ribosomal RNA (r-RNA)
It is a component of ribosomes which are the sites for protein biosynthesis
- Heterogenous nuclear RNA (hnRNA)
It is the primary form of RNA in nucleus processed into mature m-RNA.
Messenger RNA (m-RNA):
-
- Messenger RNA is synthesised as the primary transcript from the template strand of DNA and later processed to m-RNA.
- 5’ capping
- Poly ‘A’ tail
- Heterogeneous nuclear RNA (hnRNA)
Transfer RNA (t-RNA)
Transfer RNA is the adapter molecule that translates the information carried by m-RNA into specific sequences of amino acids.
tRNA contains mainly four arms
- Acceptor arm
- Anticodon arm
- D arm
- T Y C arm
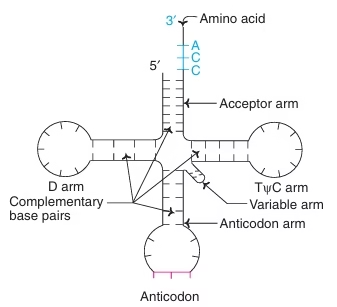 Fig: Structure of t-RNA
Fig: Structure of t-RNA
Differences between DNA and RNA:
| DNA | RNA | |
| Pentose Sugar | Deoxyribose | Ribose |
| Pyrimidine Base | Thymine | Uracil |
| Base Pairing | A = T
G ≡ C |
A = U
G ≡ C |
| Structure | Double stranded polynucleotide | Single stranded
polynucleotide |
| Chargaff’s rule | Obeyed (Content of purine equals to pyrimidine A = T,
G =C) |
Not obeyed (Single stranded, no specific relation between purine and pyrimidines) |
| Alkali hydrolysis | Susceptible (due to presence of hydroxyl group at 2’ position) | Not susceptible (Absence of hydroxyl group at 2’ position) |
| Orcinol colour reactions | DNA cannot be identified | RNA can be identified by orcinol colour reactions due to presence of ribose |
Gout
- Gout occurs in 3 out of 1000 persons and is more common in men & postmenopausal women.
- In ECF, where sodium is the major cation, uric acid occurs in combination with Na+ as its salt solution, monosodium urate. It is minimally soluble and ECF is easily saturated with monosodium urate just above the normal level.
- Monosodium urate crystals are deposited in the synovium and synovial fluid of the joints causing excruciating joint pain associated with swelling and redness. This inflammatory condition is known as ‘gout’ (gouty arthritis).
- In addition to inflammatory arthritis, as the crystal deposits slowly increase, they accumulate in the soft tissues and is known as tophi. Sometimes gout is associated with formation of uric acid renal stones (urolithiasis).
- Monosodium urate salt is minimally soluble and hence can be easily precipitated at lower temperatures. Since the intra-articular temperatures of the peripheral joints (small joints of hands and feet) are relatively lower, they are the most commonly affected. The first MTP joint (the great toe) is the classical site affected by gouty arthritis.
Primary Gout:
- Either idiopathic or a result of inborn errors of metabolism.
- Under excretion of uric acid
- Diet rich in purines/alcohol; deficient in dairy products
- Increased purine degradation
- Increased PRPP Synthetase activity
- Decreased/partial HGPRT activity
- HGPRT deficiency
- Glucose-6-phosphatase deficiency
- Abnormal variant of glutathione reductase
Secondary gout:
- Secondary to certain clinical conditions causing increased synthesis or decreased excretion of uric acid.
- Due to increased cell growth and turnover, increased degradation of nucleotides: Mallignancies (leukemia, lymphoma, myeloma) psoriasis and chronic alcoholism.
- Decreased uric acid disposal: Chronic renal disease, lead poisoning.
Diagnosis:
Identification of urate crystals in joint fluid or the presence of tophi is diagnostic of gout.
Treatment of Gout:
Allopurinol:
- Drug of choice in the treatment of gout.
- It is a structural analogue of hypoxanthine and acts as a competitive inhibitor of Xanthine oxidase, thus decreasing the formation of uric acid.
- Xanthine and hypoxanthine are more soluble than uric acid and are easily excreted.
- Xanthine oxidase converts Allopurinol to alloxanthine which is more potent inhibitor of xanthine oxidase than Allopurinol. This is suitable example of suicide inhibition.
Uricosuric Drugs: (Ex Probencid) decrease the reabsorption of uric acid by renal tubules and thereby, increase the excretion of it.
Anti-inflammatory drug: Colchicine, phenylbutazone, indomethacin
Lesch–Nyhan syndrome
- This genetic disorder is characterised by spasticity, mental retardation, self-injurious behaviour and It was first described in 1964 by a medical student, Michael Lesch, and his professor, William Nyhan.
- Inherited deficiency of the purine salvage enzyme, hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is responsible for the Lesch–Nyhan syndrome.
- X-Linked recessive disorder. It affects only males.
- Complete HGPRT deficiency
- The affected children apart from exhibiting the signs and symptoms of gout, show mental deficiency, aggression, and compulsive self-destructive behavior, characterized by irresistable urge to bite their lips and fingers often causing self-mutilation.
- Since high urate levels are present in the blood, individuals with this condition are also prone to gout and kidney stones.
- Allopurinol is used to treat hyperuricemia, but it has no effect on the neurological manifestations on these patients.
Severe Combined Immunodeficiency (SCID):
Deficiency of the enzyme adenosine deaminase (ADA).
Involving both T-cell and B-cell dysfunction.