
Definition
Carbohydrates are polyhydroxy aldehydes or ketones or compounds which yield these on hydrolysis.
Functions of Carbohydrates:
- Most abundant dietary source of energy (4 cal/gm).
- Storage form of energy in the form of Glycogen.
- Structural components of many organisms.
- Participate in structure of cell membrane.
- Precursors of organic compounds (fats, Amino Acids)
- Joint lubrication and cartilage formation
Classification
Broadly classified into 3 groups
- Monosaccharides
- Oligo saccharides
- Polysaccharides
Monosaccharides:
- Simple sugars which can’t be hydrolyzed further.
- Soluble in water & sweet to taste.
- Classified depending upon-
- Number of carbon atoms present:
Trioses(3C), Tetroses(4c), Pentoses(5C), Hexoses(6C) & Heptoses(7C).
- Functional group present-
Aldoses-when aldehyde (CHO) group present
Ketoses-when ketone(C=O) group present.
| Aldoses | Ketoses | |
| Triose (3c) | Glycerldehyde | Dihydroxyacetone |
| Tetrose (4c) | Erythrose | Erythrulose |
| Pentose (5c) | Riboses | Ribulose |
| Hexose (6c) | Glucose | Fructose |
| Heptose (7c) | Glucoheptose | Sedoheptulose |
Biological Importance of Monosaccharides:
| Glyceraldehyde | Glyceraldehyde-3-phosphate is an intermediate in glycolysis |
| Dihydroxyacetone | Dihydroxyacetone-1-phosphate is an intermediate in glycolysis |
| Ribose | For the structure of RNA and nucleotide coenzymes (ATP, NAD+, NADP+) |
| Deoxyribose | For the structure of DNA |
| Ribulose | Important metabolite in HMP shunt |
| Xylose | Involved in function of glycoproteins |
| Glucose | ‘sugar fuel of life’ , Structural unit of cellulose in plants |
| Mannose | For the structure of polysaccharides |
| Fructose | Its phosphates are intermediate in glycolysis |
Oligosaccharides:
- 3-10 Monosaccharide units joined with glycosidic linkage.
- Disaccharides: Lactose, Maltose, Sucrose
- Trisaccharides : Raffinose (α-D-glucose, β-D-galactose, β-D-fructose)
- Tetrasaccharides : Stachyose (Galactose + Galactose + Glucose + Fructose)
Disaccharides:
Contain two monosaccharide units joined together by a Glycosidic bond.
Sucrose- Glucose & Fructose
Lactose- Galactose & Glucose
Maltose- Two Glucose units.
Two types:
-
- Reducing Disaccharides:- Free aldehyde or keto group
Eg. Maltose, Lactose.
-
- Non – reducing Disaccharides:- No free group.
Eg. Sucrose, Trehalose.
Maltose-
- Two α-D-glucose units held together by α(1→ 4) glycosidic bond.
- Produced by digestion of starch by pancreatic amylase.
- Osazone formation sunflower shaped crystals.
Lactose-
- Most important carbohydrate in nutrition of young mammals (Milk sugar).
- β-D-galactose & β-D-glucose held together with β(1 → 4) glycosidic bond.
- Hydrolysed by enzyme lactase
Sucrose–
- Cane sugar.
- α-D-glucose & β-D-fructose held together by glycosidic bond (α1 → β2)
- Major carbohydrate produced in photosynthesis.
- Sweeter than most other common sugars (except Fructose).
- Intestinal enzyme Sucrase hydrolyses it.
Polysaccharides:
Consist of repeating units of monosaccharide units or derivatives ( > 10) held together by glycosidic bond.
Two types-
-
- Homopolysaccharides– Example: Starch, Glycogen, Cellulose
On hydrolysis single type of monosaccharides.
Glucans are polymers of Glucose & Fructosans are polymers of Fructose.
Starch–
- Carbohydrate reserve of plants & most important dietary source for higher animals including man.
- Consist of D-Glucose units held together with α-glycosidic bonds & known as glucosan or glucans.
- Two polysaccharide components- water soluble amylose (15-20℅) & water insoluble amylopectin (80-85℅).
Dextrins-
Breakdown products of Starch by enzyme amylase or dilute acids.
Inulin–
- Polymer of Fructose i.e. Fructosan.
- Used for assessment of GFR (Glomerular Filtration Rate)
Glycogen–
- Carbohydrate reserve of animals, hence called animal starch.
- Highest concentration in liver, muscle & brain.
- Repeating units of Glucose with α(1→4) bond & α(1→ 6) bond at branching site.
- Structure similar to amylopectin with more number of branches and more compact.
Cellulose-
- Occurs extensively in plants & it is totally absent in animals.
- Unbranched linear; repeating units of β-D-Glucose linked by β(1→ 4) bonds.
- Not digested by mammals due to lack of enzyme that cleaves β glycosidic bond.
- Major constituent of dietary fibre
- Heteropolysaccharides– Example: Hyaluronic acid
On hydrolysis yield a mixture of few monosaccharides or their derivatives.
- Repeating units of amino sugars & uronic acid.
- More commonly known as Glycosaminoglycans (GAG).
- Ground substance of extracellular matrix is mainly composed of GAGs.
- Hyaluronic acid-
- Found in synovial fluid of joints & vitreous humor of eyes.
- Present in connective tissues and gel around ovum.
- Lubricant and shock absorbent in joints.
- Heparin-
- Anticoagulant; occurs in blood, lung, liver, kidney.
- Helps in release of lipoprotein lipase clearing lipemic plasma.
- It prevents intravascular clotting in thromboembolisms.
- Chondroitin sulfates-
- Major constituent of bone, cartilage, tendons, heart valves.
- Contributes to the compressibility and weight bearing capacity of the cartilage.
- Dermatan sulfate-
- Mostly occurs in skin & sclera.
- Responsible for maintaining shape of the eyeball.
- Keratan sulfate-
- Occurs in cornea, tendons, cartilage.
- Role in corneal transparency.
Digestion and absorption of carbohydrates
Answer:
- Process involving hydrolysis of large and complex organic molecules of foodstuffs into smaller and preferably water-soluble molecules which can be easily absorbed by the gastrointestinal tract for utilization by the organism. It is in the form of these smaller molecules produced by digestion that the food moves from lumen of the gut across the membranes of epithelial cells into blood or lymph. This process is known as absorption.
- Cooking of the food and its mastication in mouth facilitates digestion. Once absorbed, the molecules are distributed to all the cells in the body by absorption.
Principal Carbohydrates:
- Dietary carbohydrates principally consist of polysaccharides (Starch and Glycogen). Ex: Wheat, Jowar, Rice, Potato, animal foods etc.
- It also contains disaccharides. Ex: Sucrose-cane sugar, lactose-milk sugar, maltose-malt sugar.
- In small amounts monosaccharides like fructose and pentose. e.g fruits, honey.
Digestion in mouth:
- Digestion of carbohydrates starts at the mouth, where they come in contact with saliva during mastication.
- Saliva contains a carbohydrate splitting enzyme called salivary amylase (ptyalin).
- Liquid food materials like milk, soup, fruit juice escape digestion in mouth as they are swallowed.
- Carbohydrates are the only nutrients whose digestion begins in mouth.
- Action of salivary amylase :hydrolyses µ,1→4 glycosidic linkage
- Starch, µ-amylase Glucose, maltose
Glycogen & maltotriose
Dextrin
Digestion in stomach:
- pH does not favour salivary amylase activity.
- Salivary amylase is inactivated by high acidity (low pH) in the stomach.
Digestion in small intestine:
Digestion in Duodenum
- Pancreatic Amylase hydrolyses µ(1→4) glycosidic linkage at random deep inside the polysaccharide molecule producing smaller molecule.
- Acidic dietary contents neutralized by bicarbonate produced by pancreas, on reaching small intestine
Digestion in Jejunum:
-
- Intestinal amylase:
Hydrolyzes terminal µ,1→4 glycosidic linkage, liberating free glucose units.
-
- Lactase:
It is a β-galactosidase. Hydrolyses Lactose → glucose + galactose.
-
- Isomaltose:
It catalyzes hydrolysis of µ,1→6 glycosidic linkage, thus acting at branching point.
-
- Maltase:
It catalyzes hydrolysis of µ,1→4 glycosidic linkage between glucose units in maltose.
Maltose→ glucose+ glucose.
-
- Sucrase:
Sucrose→ glucose + fructose.
Digestion in Small intestine:
- Final digestion of di- and oligo- saccharides to monosaccharides occurs at mucosal lining of upper jejunum.
- On mucosal linings of upper jejunum, oliosaccharidases & disaccharidases are present.
Absorption of monosaccharides:
- Mainly in duodenum & upper jejunum.
- Rate of absorption diminishes from above downwards ; proximal jejunum three times greater than that of distal ileum.
- Galactose > glucose > fructose > mannose > xylose > arabinose
- 110 100 43 19 15 9
- Galactose is most efficiently absorbed.
Mechanism of Absorption:
- Simple passive diffusion: All the monosaccharides are absorbed by simple passive diffusion to some extent. Mainly pentoses.
- Facilitated diffusion: Fructose.
- Secondary Active transport: Glucose and galactose. Na-K pump.
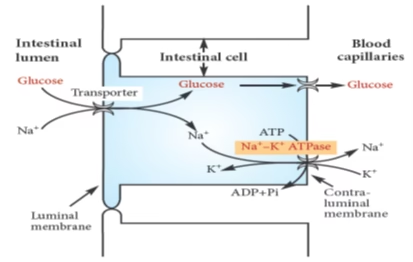 Fig: Transport of glucose across intestinal epithelium
Fig: Transport of glucose across intestinal epithelium
Abnormalities of carbohydrates digestion
- Disaccharidase deficiency – Osmosis & osmotic diarrhea.
- Lactose intolerance– Lactase (β-galactosidase) deficiency
- It is the most common disaccharidase deficiency. There is reduced production of lactase rather than reduction in enzyme activity. Flatulence is most common feature. Increased intestinal motility, cramps and irritation. After ingestion of leguminous seeds.
- Primary (congenital) or secondary (acquired)
- Acquired due to sudden and high intake of milk-based diets..
- Lactose intolerance is basically due to the deficiency of the enzyme lactase (b-galactosidase). Normally, lactase hydrolyses lactose into galactose and glucose. As lactase activity is also rate-limiting for lactose absorption, deficiency of the enzyme hinders the digestion and absorption of lactose in the gut.
- Signs and symptoms of lactose intolerance include diarrhoea, flatulence, abdominal cramps, irritability etc.
- Treatment:
- By feeding a lactose free diet to the affected infants and avoidance of lactose rich foods (milk and dairy products) in adults. Synthetic preparations of b-galactosidase (lactase) are also available.
- Sucrase deficiency also causes similar symptoms.
Non-digestible carbohydrates:
- Fibers- chemically complex carbohydrates.
- Cellulose, hemicellulose, pectins, lignins, gums.
Cannot be digested by human enzymesor intestinal bacteria.
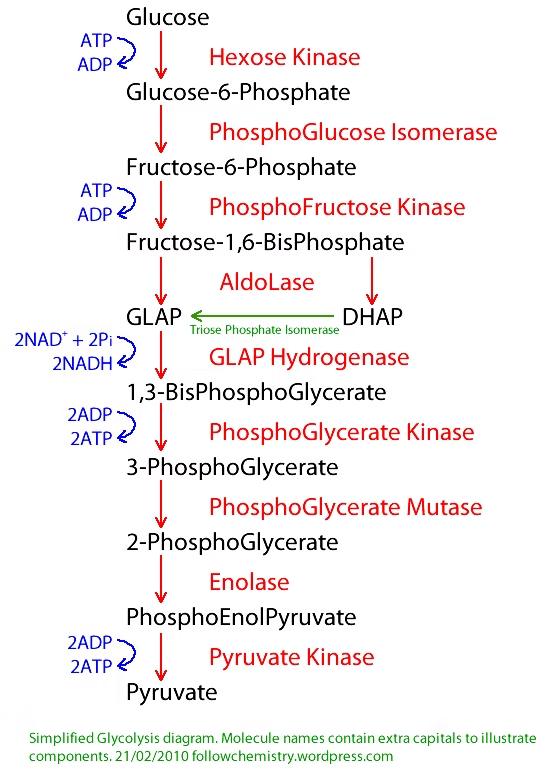
Glycolysis (Embden-Meyerhof Pathway)
- Cytosolic pathway; occurs in all cells.
- No stringent requirement of Oxygen. (aerobic/ anaerobic)
- Very essential for brain.
- Sequence of reactions converting glucose (or glycogen) to pyruvate to lactate, with ATP production.
- Definition: Oxidation of glucose or glycogen to pyruvate and lactate.
- Site: All tissues.
- Cytosolic; Enzymes are extra-mitochondrial.
- Major pathway for ATP in tissues lacking mitochondria; erythrocytes, cornea, lens
- Unique pathway (aerobic & anaerobic).
- Phases : Energy investment
Splitting
Energy generation
Conversion of Pyruvate to Lactate: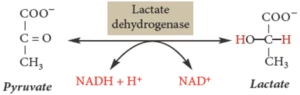
Energetics of Glycolysis:
| Condition | Enzymes | ATP |
| Aerobic | Glyceraldehyde 3 Phosphate dehydrogenase | 5 |
| Phosphoglecerate Kinase (Substrate level phosphorylation) | 2 | |
| Pyruvate Kinase (Substrate level phosphorylation) | 2 | |
| Hexo/Glucokinase | -1 | |
| Phosphofructokinase | -1 | |
| Anerobic | Pyruvate dehydrogenase | -5 |
Net ATP Generation in Glycolysis:
Aerobic – 7 ATP
Anaerobic – 2 ATP For Glycogen: 3 (anaerobic)
Inhibitors of Glycolysis:
- Glyceraldehyde 3-phosphate dehydrogenase: Iodoacetate & arsenate
- Enolase: Fluoride
Substrate level phosphorylation:
- Phosphoglycerate kinase
- Pyruvate Kinase
Regulation of Glycolysis:
Irreversible steps in glycolysis
- Hexokinase
- Phosphofructokinase (Rate limiting Committed step)
- Pyruvate kinase.
| Enzyme | Activation | Inhibition |
| HK
GK |
Insulin | G-6-P
Glucagon |
| PFK | Insulin, AMP, F6-P, PFK-2,
F2,6 BP |
Glucagon, ATP, cAMP, Citrate, H+ |
| PK | Insulin, F1,6-BP | Glucagon, ATP,
cAMP |
Hormone Regulation:
- Insulin turns on, Glucagon turns off.
- Epinephrine turns on in muscle, off in liver
Rapaport-Leubering cycle:
- Supplementary pathway to glycolysis.
- In erythrocytes of man and other mammals.
- Mainly concerned with synthesis of 2,3 BPG.
- About 15-25% of glucose that is converted to lactate in erythrocytes goes via 2,3 BPG synthesis.
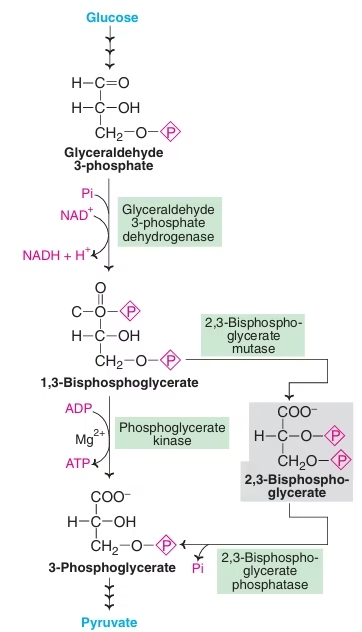
Significance of 2,3-BPG:
- Production of 2,3 BPG allows glycolysis to proceed without ATP formation. Shunt pathway to glycolysis to dissipate or waste the energy not needed by erythrocytes.
- Has high affinity for Hb, so reduces Hb affinity with oxygen and unloads more oxygen to tissues.
- Role in hypoxia- In presence of 2,3 BPG shifts oxyhemoglobin dissociation curve to right, so oxyhemoglobin unloads more oxygen to tissues.
- At high altitude, hypoxic conditions, anemia, fetal tissue: 2,3-BPG increases: Enhance the supply of oxygen to tissues.
Kreb’s cycle
Conversion of Pyruvate to Acetyl CoA:
Pyruvate Dehydrogenase complex: PDH complex (Multienzyme complex)
Five Cofactors/Coenzymes:
- TPP
- FAD
- NAD+
- CoA
- Lipoamide
Kreb’s cycle- Involved Vitamins:
- Thiamine (TPP)
- Riboflavin (FAD)
- Niacin (NAD)
- Pantothenic acid (CoA)
- Lipoic acid (Lipoamide)
Regulation of PDH: Pyruvate Dehydrogenase complex
- End product inhibition: Acetyl CoA, NADH
- Regulation by covalent modification (Phosphorylation)
- PDH active as dephosphoenzyme: Promoted by Ca2+, Mg2+, Insulin.
- PDH inactive as phosphoenzyme: Promoted by ATP, NADH, Acetyl CoA.
Biochemical Importance of PDH:
- Lack of TPP (Thiamine deficiency- Alcoholics): Accumulation of pyruvate,
In Alcoholics: Pyruvate → Lactate; Leads to Lactic Acidosis.
- Inherited deficiency of PDH: Lactic acidosis after glucose load.
- PDH inhibited by arsenic and mercuric ions.
TCA/Citric acid/ Krebs cycle:
- Definition: Oxidation of acetyl CoA to CO2 and H2 It generates 65-70% of total ATP.
- Unlike glycolysis it is only aerobic; utilizes 2/3 of total O2.
- Site: Mitochondrial matrix in close proximity to ETC.
- Central metabolic pathway/ Final common pathway for carbohydrates, fats and amino acids.
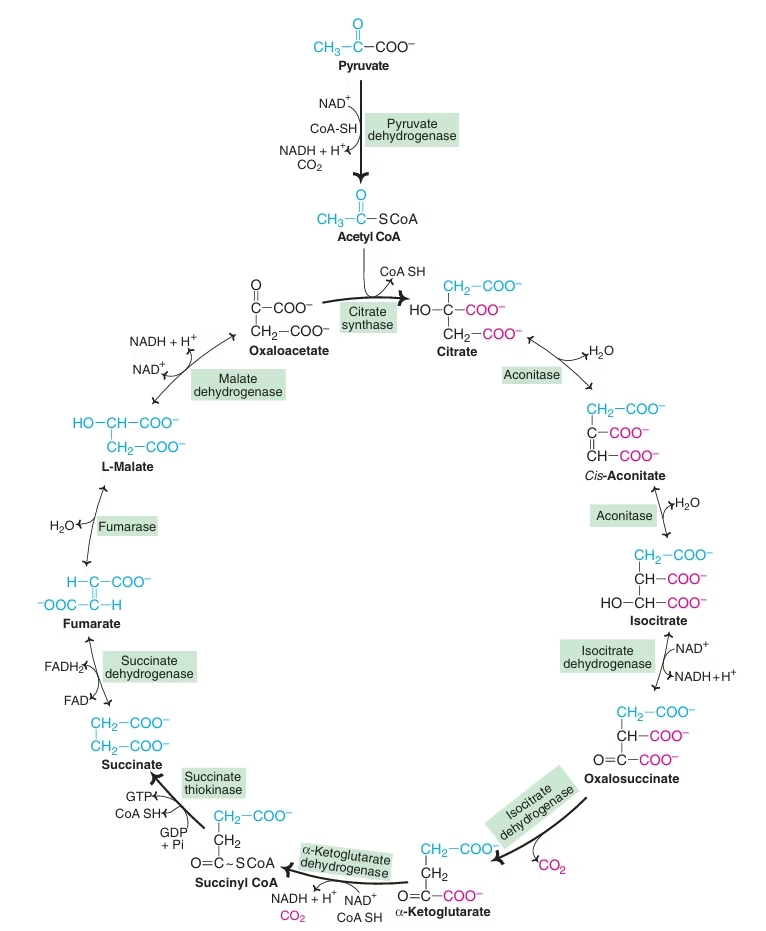 Fig: TCA cycle as common metabolic pathway
Fig: TCA cycle as common metabolic pathway
- Not only supplies energy but also provides many intermediates required for the synthesis of amino acids, glucose, heme etc.
- TCA cycle is an open cycle
- Oxaloacetate: plays a catalytic role
- Connecting almost all individual metabolic pathways.
- No net consumption of Oxaloacetate or any other intermediate.
- Tricarboxylic acids- citrate, cis-aconitate, isocitrate
Reactions of TCA Cycle;
- Synthesis of citrate
- Isomerisation of citrate to isocitrate
- Oxidative decarboxylation of isocitrate to a-ketoglutarate (a-KG)
- Oxidative decarboxylation of a-KG to succinyl CoA:
- Formation of succinate
- Oxidation of succinate to fumarate
- Formation of malate
- Conversion of malate to oxaloacetate
- α-KG dehydrogenase:
Requires TPP, Lipoic acid, CoA-SH, FAD, NAD+ & Mg++. Arsenite inhibits.
- Succinate Dehydrogenase:
FAD dependant enzyme succinate dehydrogenase is Ferri-flavo protein. Malonate/ OAA is competitive inhibitor.
-
- The net reaction of the citric acid cycle is as follows:
Acetyl CoA + 3NAD+ + FAD + GDP + Pi + 2H2O →
2CO2 + 3 NADH + 3H+ + FADH2 + GTP + CoA
-
- Substrate level phosphorylation: Succinate thiokinase
Energetics of Pyruvate to acetyl CoA:
Energetics of TCA cycle and per mole of Glucose oxidation:
Energetics of Kreb’s Cycle:
| Enzymes | ATP |
| Pyruvate dehydrogenase (2 NADH) | 5 |
| Isocitrate dehydrogenase (2 NADH) | 5 |
| Ketoglutarate dehydrogenase (2 NADH) | 5 |
| Succinate thiokinase (Substrate level phosphorylation) | 2 |
| Succinate dehydrogenase (2 FADH) | 3 |
| Malate dehydrogenase (2 NADH) | 5 |
| Total ATP per mole of glucose under aerobic | 32 (7 Glycolysis + 5 + 20 TCA Cycle) |
| Total ATP per mole of glucose under anaerobic | 2 |
| TCA Cycle Proper | 20 |
- One acetyl CoA — 10 ATP
- One pyruvate — 12.5 ATP
- One glucose — 32 ATP
Regulation of Kreb’s Cycle:
- Citrate synthase: Inhibited by ATP, NADH, acetyl CoA, succinyl CoA.
- Isocitrate dehydrogenase: Inhibited by ATP and NADH, activated by ADP.
- α-keto glutarate dehydrogenase: Inhibited by NADH and succinyl CoA.
- Availability of ADP: Unless sufficient amounts of ADP available, oxidation of NADH and FADH2 through ETC stops.
- O2 availability: NAD+ and FAD required for cycle’s operation can be regenerated in respiratory chain only in presence of O2.
Inhibitors of Kreb’s Cycle:
- Fluoroacetate – Aconitase (non-competitive)
Suicidal substrate: fluoroacetate- fluorocitrate
- Arsenite – µ-ketoglutarate dehydrogenase (non-competitive)
- Malonate – Succinate dehydrogenase (competitive)
‘Amphibolic’ nature of TCA:
TCA cycle has dual role: Catabolic and anabolic
Catabolic: Acetyl CoA → CO2 and H2O
Anabolic:
- Transamination- Non-essential amino acids.
- Formation of glucose.
- Fatty acid synthesis.
- Synthesis of cholesterol and sterols.
- Heme & porphyrin synthesis.
- Formation of purines and pyrimidines.
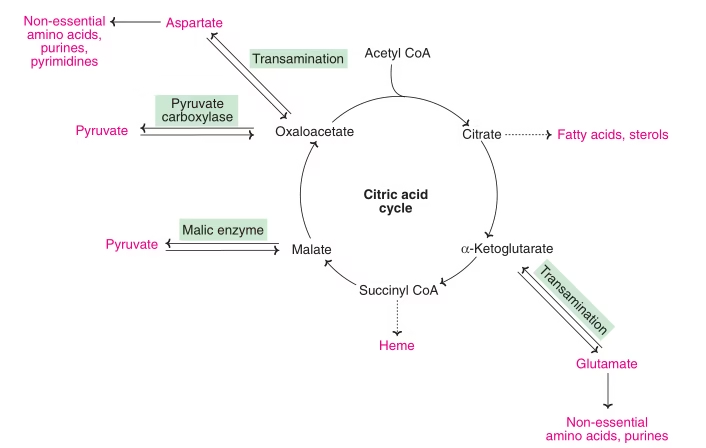 Fig: Amphibolic nature of TCA Cycle
Fig: Amphibolic nature of TCA Cycle
Anaplerotic Reactions:
- Reactions concerned to replenish or to fill up the intermediates of TCA cycle- anaplerosis
- Pyruvate Carboxylase: Pyruvate → Oxaloacetate.
- Malate Dehydrogenase: Pyruvate → Malate.
- Transamination: α-ketoglutarate and Oxaloacetate.
- Glutamate Dehyrdrogenase: Glutamate → α-ketoglutarate
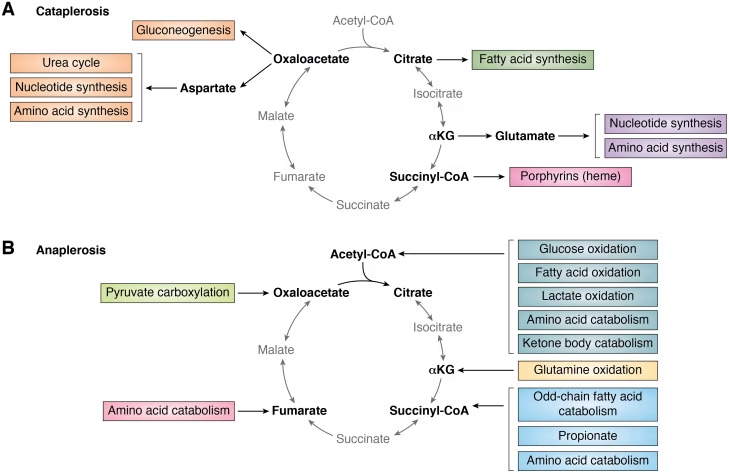 Fig: Anaplerosis or Anaplerotic reactions of Kreb’s cycle
Fig: Anaplerosis or Anaplerotic reactions of Kreb’s cycle
Gluconeogenesis
Gluconeogenesis:
- Gluco- glucose ; Neo- new; Genesis- synthesis
Definition:
- The synthesis of new glucose or glycogen from non-carbohydrate sources is called gluconeogenesis.
Site of Gluconeogenesis:
Gluconeogenesis mostly occurs in liver and to some extent, in the kidneys. With the exception of a few reactions that take place in the mitochondria, the pathway occurs in cytosol.
- Substrates for gluconeogenesis:
- Pyruvate,
- Lactate,
- Propionic acid,
- Glycerol,
- Glucogenic amino acids.
Key enzymes in Gluconeogenesis:
- Pyruvate carboxylase
- Phosphoenol pyruvate carboxykinase
- Fructose 1,6 Bis-phosphatase
- Glucose 6-phosphatase
Fructose 1,6 bis-phosphatase and Glucose 6-phosphatase: Both absent in muscles
Gluconeogenesis from Fat:
- Glycerol from Lipolysis
- Propionate from oxidation of odd chain Fatty acids.
Conversion of Propionic acid to glucose:
Conversion of Glycerol to Glucose
Cori Cycle:
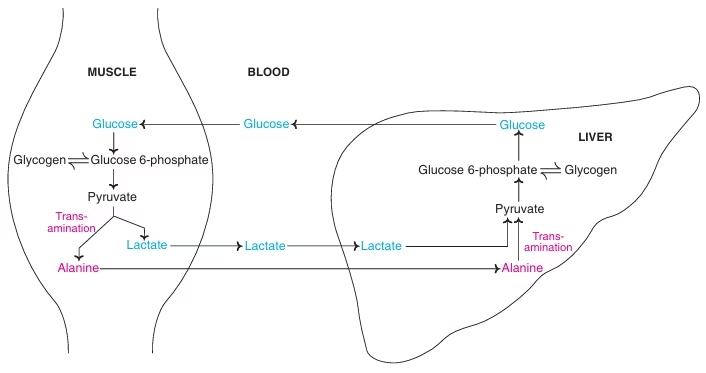 Fig: The Cori cycle (black) and glucose–alanine cycle (blue)
Fig: The Cori cycle (black) and glucose–alanine cycle (blue)
- Pyruvate + NADH + H+ ↔ Lactate + NAD+
- Lactate or pyruvate in muscle cannot be utilized for glucose synthesis due to absence of glucose 6-phosphatase & fructose 1,6 bisphosphatase.
- Synthesis of glucose in liver from skeletal muscle lactate and reuse of glucose by muscle for energy purpose is Cori’s cycle.
Glucose-Alanine cycle:
- Transport of AA (alanine dominates) from muscle to liver, mainly in starvation.
- Alanine is transported to liver and utilized for gluconeogenesis.
- Indirect way of utilizing muscle glycogen to maintain blood glucose in fasting state.
Regulation of Gluconeogenesis:.
Glucagon: activates gluconeogenic enzymes and inactivates glycolytic enzymes.
- Glucagon, cortisol and adrenalin activates all regulatory enzymes of gluconeogenesis.
- Insulin has opposite action, i.e. it inhibits
Glucagon: Activates Gluconeogenesis
- Glucagon: ↑ cAMP: activates pyruvate carboxylase and also other regulatory enzymes.
- Glucagon: ↑ cAMP: inactivates pyruvate kinase : Inhibits glycolysis.
- Phosphoenolpyruvate not coverted to Pyruvate, so diverted to gluconeogenesis.
- Glucagon: ↓ Fructose 2, 6 Biphosphate : inhibits PFK & activates Fructose 1,6 Biphosphatase.
Acetyl CoA is an activator of pyruvate carboxylase.
- Fatty acid (odd chain) oxidation promotes gluconeogenesis.
- Substrates availability induces gluconeogenesis.
In DM – AAs mobilized for gluconeogenesis.
- During starvation – Excess lipolysis – Acetyl CoA accumulates – Promotes gluconeogenesis.
Alcohol inhibits Gluconeogenesis:
- Ethanol + NAD+ → Acetaldehyde + NADH + H+
- Pyruvate + NADH + H+ → Lactate + NAD+
- Oxaloacetate + NADH + H+ → Malate + NAD+
- Substrates made unavailable
Significance of Gluconeogenesis:
- Gluconeogenesis meets the requirements of glucose.
- Glucose is the source of energy for nervous tissues and erythrocytes. Human Brain requires 120g glucose daily out of 160g required by the body.
- Other carbohydrates are synthesized from glucose. Glucose forms glycerol and hence fatty acids.
- Starvation- gluconeogenesis must occur to meet the basal requirements of the body for glucose and to maintain the intermediates of TCA.
- It serves as only fuel for skeletal muscles in anaerobic conditions.
- Gluconeogenic mechanisms are required to clear metabolic products from blood e.g. Lactate, Pyruvate, Glycerol.
Glycogen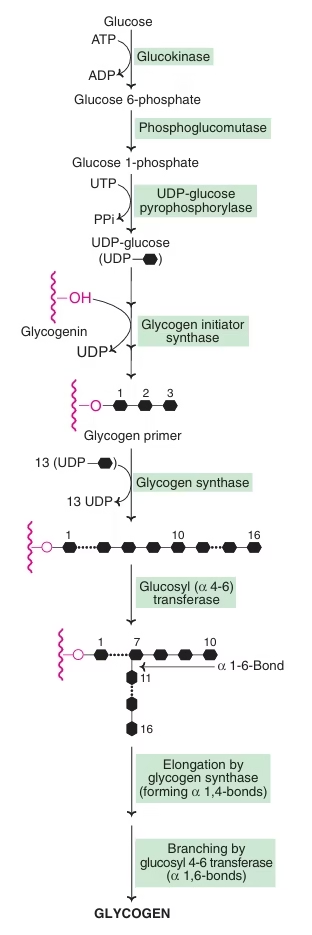
- Stored in liver(6-8%) and muscle (1-2%).
- In muscle 250gm; In liver 75gm.
- Functions:
- Liver glycogen- Maintain blood glucose levels, between meals.
- Muscle Glycogen- fuel reserve for ATP supply during muscle contraction.
Why store Glycogen as a fuel reserve?
- It is rapidly mobilized.
- It can generate energy in absence of oxygen.
- Brain depends on continuous glucose supply.
- Fats: Slowly mobilized, Need O2 for energy production.
- Fat: Fixed deposit; Glycogen: Current/savings
Glycogen Storage Diseases: GSD
- Metabolic defects concerned with glycogenesis and Glycogenolysis.
- von Gierke’s disease: Type I GSD
- Deficiency of Glucose 6 Phosphatase.
- Features:
- Hypoglycemia: Free glucose not released from liver
- Lactic Acidemia: Glucose not produced from lactate
- Hyperlipidemia: More lipolysis as glucose not available
- Hyperuricemia: G-6-P diverted to HMP shunt
| Glycogen Storage Diseases | Enzyme Defect |
| Type I GSD: von Gierke’s disease | Glucose 6 Phosphatase |
| Type II GSD: Pompe’s disease | Lysososmal alpha 1,4 glucosidase (Acid maltase) |
| Type III: Cori’s disease (Limit Dextrinosis, Forbe’s disease) | Amylo alpha 1,6 glucosidase (Debranching enzyme) |
| Type IV: Anderson’s disease (Amylopectinosis) | Glucosyl 4,6 transferase (Branching enzyme) |
| Type V: McArdle’s disease | Muscle glycogen Phosphorylase |
| Type VI: Her’s disease | Liver glycogen Phosphorylase |
Hexose Monophosphate Shunt (HMP Shunt)
- Also called as pentose phosphate pathway or phosphogluconate pathway.
- HMP Shunt is an alternative pathway to glycolysis and TCA for the oxidation of glucose.
- HMP shunt is more anabolic in nature, since it is concerned with the biosynthesis of pentoses & NADPH. It occurs in tissues which are actively involved in the biosynthesis of fatty acids and steroids.
- Tissue: Liver, adipose tissue, adrenal gland, erythrocytes, testes, lens of the eye and lactating mammary glands.
- Organelle:
- No ATP is directly utilized or produced.
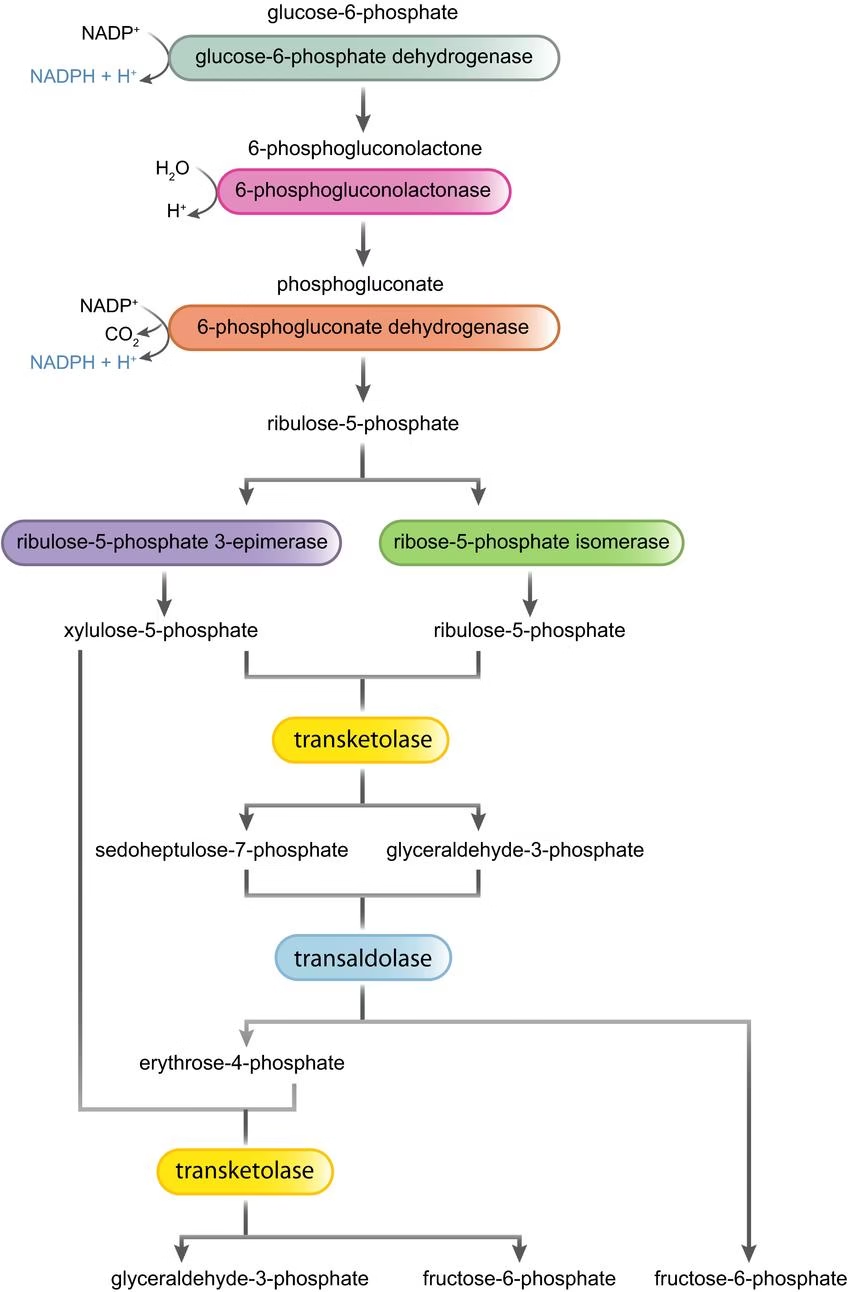
Metabolic Significance of HMP Shunt:
- Formation of NADPH.
- Provision of pentoses.
Functions of Pentose:
- Synthesis of nucleic acids: RNA, DNA.
- Synthesis of nucleotides: ATP, NAD+, FAD etc.
Functions of NADPH:
- Reductive biosynthesis of fatty acids and steroids.
- Synthesis of amino acids- glutamate dehydrogenase.
- Free radical scavenging: Antioxidant.
- Detoxification-hydroxylation. Cytochrome P450
- Phagocytosis.
- Integrity of RBC membrane.
- Lens of the eye.
- Neurotransmitter biosynthesis
Clinical aspects of HMP Shunt:
- G6PD deficiency:
- Transmitted as an X-linked recessive trait.
- Drug induced Hemolytic anaemia.
- Antimalarial (primaquine), acetanilide (antipyretic), sulfamethooxazole (antibiotic) precipitate the hemolysis- jaundice and severe anemia.
- Resistance to malaria:
- P. falcifarum, malarial parasite needs reduced glutathione for its survival.
- Wernicke –Korsakoff syndrome (encephalopathy)
- Genetic defect in transketolase activity.
- Seen in thiamine deficiency.
Blood glucose homeostasis
Factors contributing to blood glucose
- Dietary carbohydrates absorbed as glucose in the intestine
- Glucose released by glycogen breakdown from the liver
- Gluconeogenesis
Factors that remove glucose from the blood
- Utilisation by the tissues for energy
- Storage as glycogen
- Conversion of surplus glucose into fat
- In normal healthy individuals, fasting plasma glucose levels are 70–110 mg/dl.
- When the concentration exceeds the normal range, it is called hyperglycemia.
- When the values are below the normal limits it is known as hypoglycemia
- Hyperglycemia: Diabetes Mellitus, hyperadrenalism, hyperpituitarism.
- Hypoglycemia: Increased doses of insulin, tumor of β-cells.
Hormonal Regulation of Blood Glucose:
Lowers Blood Glucose (Hypoglycemic)
- Insulin
Increases Blood Glucose (Hyperglycemic)
- Glucagon
- Epinephrine
- Thyroxine
- Glucocorticoids
- Growth hormone & ACTH
Hypoglycemic effect:
Insulin
- Promotes tissue uptake of glucose
- Enhances glycolysis
- Favours glycogen synthesis
- Depresses glycogen breakdown
- Inhibits gluconeogenesis
- Inhibits glucagon release from the pancreas and depresses glucagon gene expression
- Increases HMP shunt
Hyperglycemic effect
Glucagon
- Promotes glycogen breakdown in liver
- Enhances gluconeogenesis
- Depresses glycogen synthesis
- Inhibits glycolysis
Epinephrine
- Stimulates glycogenolysis
- Decreases glucose use
- Increases gluconeogenesis
- Stimulates glucagon secretion and inhibits insulin secretion by the pancreas
Cortisol
- Stimulates gluconeogenesis
Growth hormone
- Stimulates gluconeogenesis
- Decreases glycolysis
- Antagonises insulin promoted glucose uptake
Glucose Tolerance Test:
- Individual’s response to oral glucose load.
Classical:
- Fasting ‘0’ and 5 samples collected at 30 minutes interval post meal.
- Total: 6 samples of blood & urine each.
Modern:
- Fasting ‘0’ (FBG) & 2 hrs sample (PMBG)
- Total: 2 samples of blood & urine each.
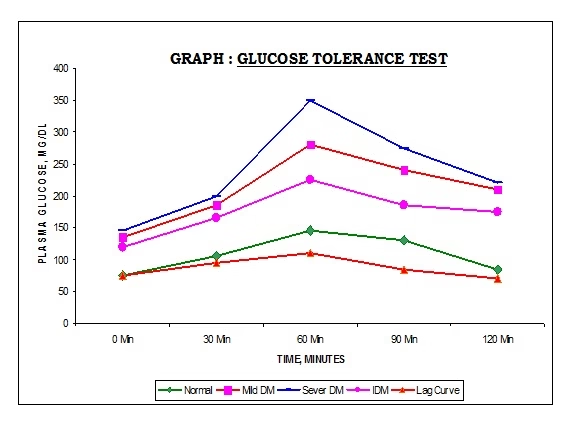 Fig: Glucose tolerance test
Fig: Glucose tolerance test
| Normal persons | Impaired Glucose tolerance (IGT) | Diabetes Mellitus | |
| Fasting | < 110 mg/dl
(< 6.1 mmol/L) |
110-126 mg/dl | >126 mg/dl
(>7.0 mmol/L) |
| 2 hours after glucose | < 140 mg/dl
(< 7.8 mmol/L) |
140-199 mg/dl | >200 mg/dl
(>11.1 mmol/L) |
Clinical Significance of Blood sugar level:
Normal levels: (by GOD-POD method)
- Fasting blood glucose (FBG) – 70-110mg%
- Post meal blood glucose (PMBG) – up to 140mg%
Hyperglycemia: Increase in blood glucose level above normal
- Diabetes Mellitus (Most common cause),
- Hyperthyroidism, hyperadrenalism, hyperpituitarism.
Hypoglycemia: Decrease in blood glucose below normal
- Increased doses of insulin,
- Tumor of β-cells.
- Hypo activity of thyroid, adrenal and pituitary glands.
- Glycogen storage diseases
Glycosuria:
- Excretion of glucose in urine.
- Renal threshold for glucose- 180 mg%.
- First line screening test for DM.
- Commonest cause- DM
Types of glycosuria:
- Hyperglycemic/ Alimentary glycosuria
- Renal glycosuria: reduced renal threshold for glucose
Test to detect glycosuria: Benedict test
Diabetes Mellitus:
- Definition: A chronic metabolic disease primarily due to a disorder of carbohydrate metabolism, cause of which is deficiency or diminished effectiveness of insulin resulting in hyperglycemia and glycosuria which lead to several systemic complications.
- Insufficient or inefficient insulin.
- Body cells are starved of glucose despite its very high concentration around (scarcity in plenty).
- Secondary changes may occur in the metabolism of proteins, fats, water and electrolytes and in tissues.
Clinical Features- Cardinal symptoms:
- Glycosuria
- Polyuria (Osmotic effect- water accompanies glucose)
- Polydypsia (Water loss- thirst centre activated)
- Polyphagia (To compensate glucose & protein loss)
- Weakness and tiredness (Tissues cannot use glucose due to absolute or relative deficiency of insulin)
- Loss of weight (Excessive breakdown of tissue proteins)
- Recurrent infections- Boils, abscesses (macrophage function inefficient)
Complication of DM:
Immediate:
- Diabetic ketoacidosis
- Hyperosmolar nonketotic coma
Late
- Atherosclerosis, MI, Stroke
- Retinopathy, Cataract
- Nephropathy
- Neuropathy, Neuritis
- Diabetic gangrene
Ketoacidosis: characterized by
- Hyperglycemia, Hyperketonemia and metabolic acidosis.
- Kussmaul’s respiration
- Sweet smell
- Hyperglycemia
- Ketosis
Diabetes mellitus is broadly classified into two categories based on etiology rather than treatment.
- Type I diabetes mellitus
- Type II diabetes mellitus
| Type I diabetes mellitusDM | Type II diabetes mellitusM | |
| Formerly known as | Insulin dependent DM | Non- Insulin dependent DM |
| Prevalence | 10-20% | 80-90% |
| Age at onset | In children <20 yrs
Juvenile onset DM |
In Adults > 35 yrs
Adult onset DM |
| Defect | Insulin deficiency due to destruction of β-cells | Impaired sensitivity of tissues to Insulin action |
| Plasma Insulin | ↓ or absent | Normal or ↑ |
| Ketosis | Common | Rare |
| Complications | Ketoacidosis | Hyperosmolar coma |
| Duration of symptom | Weeks | Months to years |
| Insulin | Required | Not necessary |
| Oral hypoglycemic | Not useful | Suitable for treatment |
Metabolic Alterations in Diabetes:
- Increased gluconeogenesis and increased Glycogenolysis: Leading to increased glucose production: Hyperglycemia and glycosuria
- Increased lipolysis: Increased fatty acids oxidation: Increased Acetyl CoA: Oxaloacetate is unavailable for combining with acetyl CoA: Impaired TCA cycle- Acetyl CoA is diverted for KB synthesis: Ketoacidosis
- Increased triglyceride synthesis: Hypertriglyceridemia and Hypercholesterolemia
- Decreased protein synthesis
- Glycosylation of Hb and formation of glycosylated Hb.
- Decrease Glycogen synthesis
Sorbitol / Polyol Pathway:
- In uncontrolled DM: Sorbitol Dehydrogenase SDH in lens, retina, nerve cells & kidney is low in activity or absent.
- Sorbitol: hydrophollic: swelling of the cells: Cataract, peripheral neuropathy, nephropathy
Investigations: Diabetic Profile
- Plasma glucose: Fasting & Postmeal
- Lipid profile- TC, TG, VLDL, LDL, HDL
- Kidney function tests- Urea & Creatinine
- Microalbuminuria : 30-300 mg/day albumin
Predicts impairment in renal function.
- Glycated hemoglobin HbA1C :
Directly related to exposure of RBC to glucose: Indicates blood glucose concentration over 6-8 weeks (half life of RBC).
Management:
- Diet & exercise:
Low calories (Less carbohydrates & fats),
Complex carbohydrates instead of simple.
High protein & fiber rich
- Oral hypoglycemic drugs
- Insulin Injections
- Management of complications.