Definition
Enzymes are Biocatalysts (the catalysts of life). They are protein in nature, colloidal and thermo-labile in character and specific in their action.
Catalyst: A substance that increases the velocity or rate of a chemical reaction without itself undergoing any change in the overall process.
Enzyme Action:
- Substrate (S): The substance on which the enzyme acts.
- Product (P): The enzyme will convert S into product.
- E + S ↔ ES → E + P
- Enzyme is Regenerated
Enzyme Classification
Location of action
- Endoenzyme: Intracellular;
Most enzymes of the metabolic pathways.
- Exoenzyme: Extracellular;
Break down (hydrolyze) large food molecules or harmful chemicals.
Example: cellulase, amylase, penicillinase
Trivial names: Originally given names to the enzymes depending on
- Type of reaction catalyzed: Dehydrogenase, protease, isomerase
- Nature of substrate: lipase, nuclease, lactase
They failed to give complete information of enzyme reaction but commonly used.
IUBMB System of Enzyme Classification:1964
International Union of Biochemistry and Molecular Biology (IUBMB) Name:
- Each enzyme given 4 digit enzyme commission (E.C.) number
First digit : the class
Second digit : subclass
Third digit : sub-sub class
Fourth digit : number of the particular enzyme
Example: Hexokinase (Trivial name)
ATP:D-hexose-6-phosphotransferase EC.2.7.1.1. (IUBMB name)
IUB names: Specific, unambiguous & informative; But lengthy, complex & cumbersome.
- Six major classes of Enzymes: OTHLIL
| Name of the Class | Role | Example |
| Oxidoreductases | H+ (electrons) transfer; Oxidation-reduction reactions | Alcohol Dehydrogenase, Lactate dehydrogenase |
| Transferases | Transfer of groups (Amino, methyl) other than hydrogen | Hexokinase, Transaminase |
| Hydrolases | Cleavage of substrate by addition of water | Lipase, Urease |
| Lyases | Cleave without adding water; removal of water, ammonia, CO2 | Aldolase, Histidase |
| Isomerases | Isomerization reaction; Intramolecular transfer | Phosphohexose isomerase |
| Ligases | Synthetic reactions; ATP dependant condensation of two molecules | Pyruvate carboxylase, Glutamine synthetase |
Chemical Nature of Enzyme
- Protein in nature (Except- RNA acting as Ribozyme).
- Tertiary structure of protein & specific conformation
- Non-protein: Coenzyme, Cofactor, Prosthetic group
- Holo enzyme → Apo enzyme + Coenzyme
(active) (protein) (non-protein)
Non Protein Moiety:
- Coenzyme – Dialyzable, organic
- Cofactor – Transient, dissociable
- Prosthetic group – Tight (covalent) binding
Structure of enzyme
Monomeric enzyme:
- Single polypeptide unit (subunit)
- Ex: Ribonuclease, trypsin
Oligomeric enzyme:
- More than one polypeptide chains (subunit)
- Lactate Dehydrogenase, Aspartate transcarbamoylase
Multienzyme complexes:
- Catalyse different reactions in a sequence.
- Pyruvate dehydrogenase, Fatty acid synthase
Cofactor
An inorganic molecule or a metal ion that certain enzymes need in order to catalyze a reaction.
Activator: Inorganic Co-factor necessary to enhance enzyme activity.
Examples of Cofactor & Activator: Cu++, Mg++, Mn++, Zn++
Metallo-Enzymes: These enzymes require certain metal ions for their activity.
| Metal | Metallo-Enzymes |
| Mg ++ | Hexokinase, Enolase, Phosphofructokinase |
| Mn ++ | Hexokinase, Enolase, Phosphoglucomutase |
| Zn ++ | Carbonic anhydrase, Alcohol dehydrogenase |
| Cu ++ | Cytochrome oxidase, superoxide dismutase |
| Fe ++ | Cytochrome oxidase, Catalase, peroxidase |
Coenzymes
- Non-protein, organic, Low Molecular Weight, dialysable substance associated with enzyme function.
- Essential for biological activity of enzyme.
- Group transfer agents: Transfer of atoms or groups (hydrogen, aldehyde, keto, amino, methyl)
- Do not decide enzyme specificity. It depends on apoenzyme.
- Act as second substrate or Co-substrate: Affinity with enzyme comparable with substrate.
- Undergo alterations during enzymatic reactions, later regenerated,
Coenzymes- Classification:
- Group I: Transfer of H+ ions or electrons
NAD+, NADP+, FAD, FMN
- Group II: Transfer of groups other than protons
ATP (Phosphate); Biotin (CO2); Coenzyme A (Acyl)
THF (One carbon group); Pyridoxal phosphate (Amino)
Vitamin Coenzymes:
| Coenzyme | Vitamin from which it is derived | Atom or group transferred |
| Thiamine pyrophosphate (TPP) | Thiamine (B1) | Aldehyde or Keto |
| FMN, FAD | Riboflavin (B2) | Hydrogen and Electron |
| NAD, NADP | Niacin (B3) | Hydrogen and Electron |
| Pyridoxal phosphate (PLP) | Pyridoxine (B6) | Amino |
| Tetrahydrofolate (FH4) | Folic acid | One carbon units |
| Biocytin | Biotin | CO2 |
| Coenzyme A (CoA) | Pantothenic acid | Acyl |
Non-Vitamin Coenzymes: ATP, CDP, UDP, SAM, PAPS
Nucleotide coenzymes: NAD+, NADP+, FMN, FAD, coenzyme A
Pro-Enzymes or Zymogens
- Inactive form of an Enzyme
- Undergo irreversible covalent activation.
- By Proteolytic trimming (breakdown of one or more peptide bonds)
- Chymotrypsinogen, Pepsinogen, plasminogen (Proenzymes) → Chymotrypsin, Pepsin, Plasmin (Active enzymes)
Enzyme Action
- Substrate (S): The substance on which enzyme acts.
- Product (P): The enzyme will convert S into product.
- E + S ↔ ES → E + P
- Enzyme is Regenerated
Active Site/ Active centre:
- Small region of big enzyme at which substrate binds and participates in catalysis.
- Substrate binding site & catalytic site
- Coenzymes & cofactors – part of catalytic site
- It is made of amino acids (catalytic residues)
- ‘Three point attachment’ of substrate by weak non-covalent bonds
- Flexibility of enzyme: Active site is not rigid.
- It exists due to tertiary structure of protein.
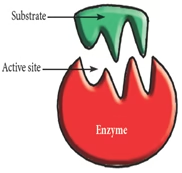 Fig: Enzyme and its Active Site
Fig: Enzyme and its Active Site
Specificity of Enzyme Action
- Ability of an enzyme to discriminate between two competing substrates.
- Most of the enzymes are highly specific in their action.
- Characteristic property of the active site of enzyme.
Significance:
- Enzymes can coexist without interfering in each other’s actions.
- Occurrence of thousands of enzymes in the biological system.
Broadly Specificity is of two types: Substrate & Reaction
Substrate Specificity is of three Types: Absolute, Group & Stereospecificity
Substrate- Absolute Specificity:
Act on only one substrate and catalyse only one reaction.
Glucose →→→ → Glucokinase →→→→→ Glucose-6-Phosphate
Lactose →→→→ Lactase →→→→→→→ Glucose + Galactose
Substrate- Group Specificity:
Found with enzymes which require specific bond with a specific group on one side.
Examples:
1) Phosphatases hydrolyses when ester bond and phosphate group are there.
2) Proteolytic enzymes:
Exopeptidases- Carboxypeptidases and Aminopeptidases
Endopeptidases- Pepsin, Trypsin and Chymotrypsin
Substrate- Stereo Specificity
- Acts on only one type of isomer.
- Ex:L-lactate dehydrogenase will act only on L-lactic acid.
- Ex: D- amino acid oxidase will act on D-amino acids.
- Class isomerase: No stereospecificity
Reaction Specificity:
Same substrate- Different reactions- Separate enzymes
Actetyl CoA PDH Pyruvate LDH Lactate
Pyruvate Transaminase
carboxylase
Oxaloacetate Alanine
Mechanism of Enzyme Action
- Ground state: Starting point of the reaction
- Transition state: Transient phase
Breaking and making of bonds take place.
- Activation energy:
Energy required by the reactants to undergo the reaction.
Difference between energy levels of ground state and Transition state.
Reactants when heated attain Activation energy.
- In uncatalysed reaction,
- High activation energy – Less no. of molecules with sufficient energy – Rate of reaction is slow.
- Enzymes: Prime function : Catalysis
- Binding energy resulting from weak non-covalent interactions between S & E, reduces the activation energy
- Do not alter the equilibrium constants.
- Lower activation energy (energy barrier).
- Enhance the velocity of reaction
- Reaction proceeds at lower temperature.
- In metabolic pathways with several steps, overall rate of reaction is determined by the step with the highest activation energy- Rate limiting step.
Enzyme-Substrate complex formation:
- Intermediate or transient complex
- Prime requisite for enzyme catalysis
- Weak non covalent bonds
- E + S ↔ ES → E + P
Theories for E-S Complex formation:
- Fischer’s template theory (Lock & key model)
- Active site: Rigid & pre-shaped where only specific substrate can bind as key fits into lock.
- Limitations:
- No scope for enzyme flexibility;
- Fails to explain allosteric effectors & inhibitors.
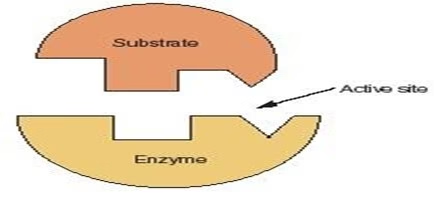 Fig: Lock & Key model
Fig: Lock & Key model
- Induced fit theory (Koshland’s model):
- Initial binding of S with E, induces a conformational change in E resulting in formation of complementary S binding site.
- Experimental evidence: X-ray diffraction studies
- Explains allosteric effectors and inhibitors along with reversible nature of enzyme catalysis.
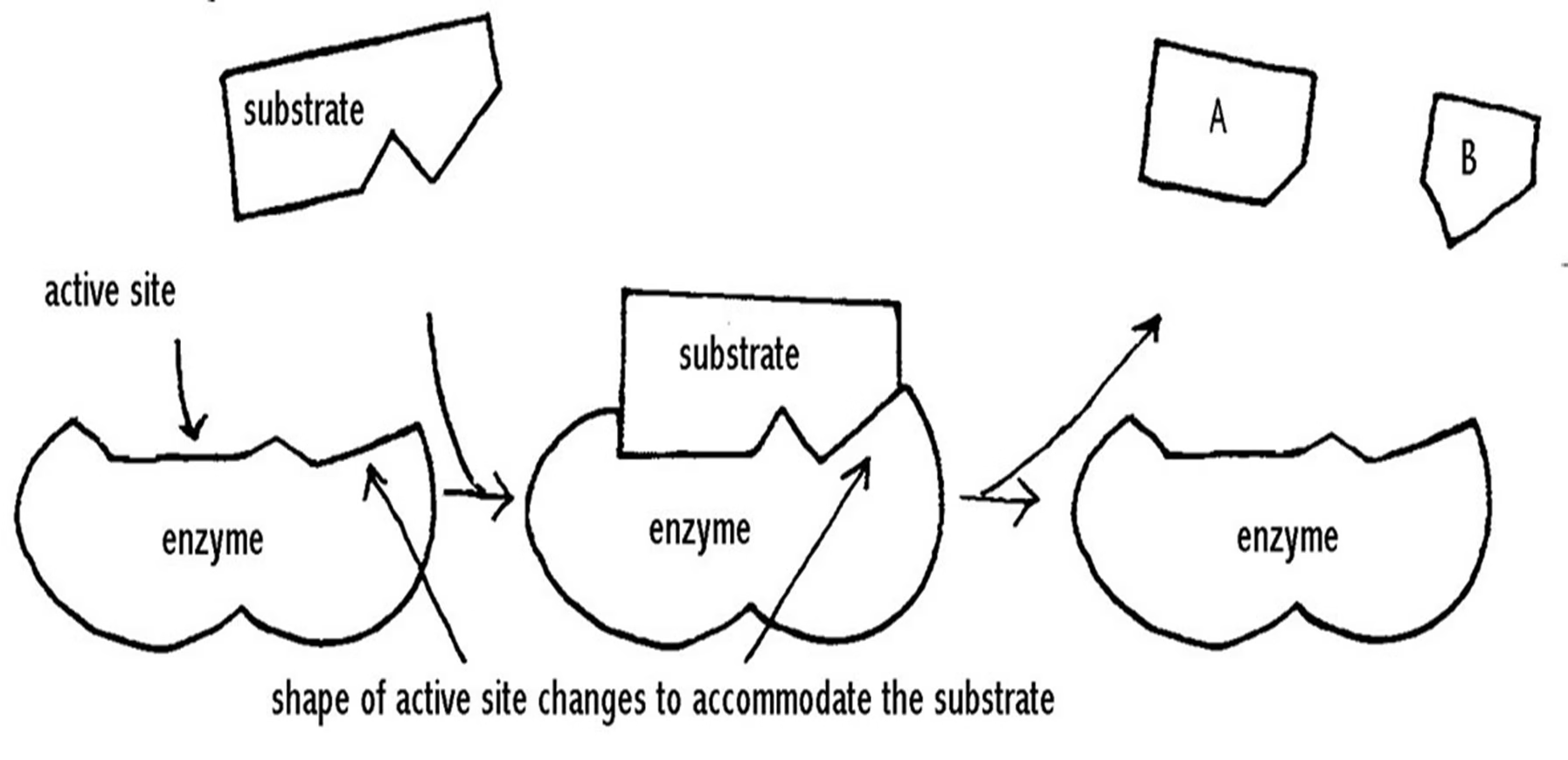 Fig: Induced fit theory
Fig: Induced fit theory
- Substrate strain theory:
- Substrate is strained due to induced conformational change in the enzyme.
- Strained substrate → distortion of bonds → transition state→ product
- Combination of induced fit with substrate strain– Enzyme Action.
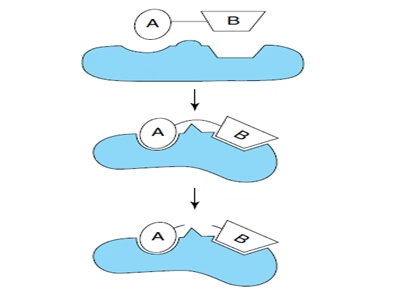 Fig: Substrate strain theory
Fig: Substrate strain theory
Factors affecting enzyme activity
- Concentration of enzyme
- Concentration of substrate
- Effect of temperature
- Effect of pH
- Effect of product conc.
- Effect of activators
- Effect of time
- Effect of light & radiation
- Concentration of enzyme:
- Velocity of reaction is directly proportional to enzyme concentration.
- Determining the level of particular enzyme in plasma, serum or tissues.
- Known volume of serum is incubated with substrate for a fixed time, then reaction is stopped and product is quantitated (Endpoint method).
- Product formed will be proportional to enzyme concentration, so it can be assessed.
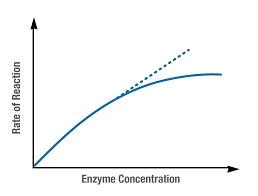 Fig: Effect of Enzyme concentration on enzyme velocity
Fig: Effect of Enzyme concentration on enzyme velocity
- Concentration of substrate:
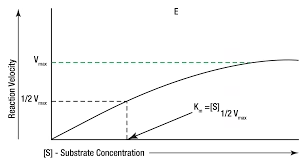 Fig: Effect of substrate concentration on enzyme velocity
Fig: Effect of substrate concentration on enzyme velocity
At low concentration of substrate, the velocity of reaction is first order that is- it is proportional to substrate concentration.
At high concentration of substrate, the velocity of reaction is zero order that is – it is constant and independent of substrate concentration.
The rate of an enzyme catalysed reaction increases as the substrate concentration increases until it reaches a maximum rate, which remains constant despite further increase in substrate concentration.
Increase in [S] : Gradually increases ‘V’ within limited range of [S].
Rectangular Hyperbola
The relationship between substrate concentration (S) and enzyme velocity (V) can be shown as follows:
When the measured velocity (V) is exactly one-half Vmax
Vmax- Maximum Velocity:
-
- The rate of an enzyme catalyzed reaction increases (V) as the substrate concentration [S] increases until it reaches a maximal rate (Vmax), which remains constant despite increase in [S].
- At maximum velocity (Vmax), enzyme is the form of ES complex.
- Vmax is achieved when E becomes saturated with S; that is, active site of every E molecule is saturated with S.
Michaleis-Menten Constant:
-
- Substrate concentration to produce half the maximum velocity in an enzyme catalysed reaction.
- Km- Brig’s & Haldane’s constant.
- Half of the enzyme molecules are bound with substrate molecules when substrate concentration equals Km.
- In Km, K stands for a constant and m stands for Michaelis and M
Significance: Km
-
- Km is signature of the enzyme. Km value is thus constant for an enzyme.
- It is the characteristic feature of particular enzyme for specific substrate.
- Measures strength of ES complex & denotes the affinity of enzyme for the substrate.
- Low Km: High affinity E-S
- High Km: Low affinity E-S
- Affinity of enzyme towards its substrate is inversely related to its dissociation constant
- Effect of Temperature:
- Bell-shaped curve: The velocity of reaction increases with increasing temperature of the medium until it reaches a maximum speed and then it falls.
- Optimum temperature: Temperature at which maximum amount of S is converted to product. (40˚C-45˚C)
- Denaturation above 50˚C
- Inactive above 70˚C
Temperature coefficient or Q10
- Increase in enzyme velocity when temperature is increased by 10 ˚C.
- Q10 is 2 between 0˚C & 40˚C
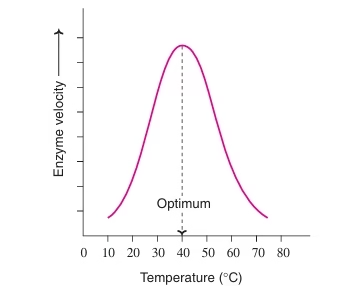 Fig: Effect of temperature on Enzyme velocity
Fig: Effect of temperature on Enzyme velocity
- Effect of pH
- Bell-shaped curve: Metabolic reactions are highly sensitive to the hydrogen ion concentration of the body fluid in which they occur. Hydrogen ions influence the enzyme function by altering the ionic charges on the amino acids at the active site.
Optimum pH:
- Most human Enzymes: Neutral pH (6-8).
- Except: Pepsin (1-2), acid phosphatase (4-5), alkaline phosphatase (10-11)
- Below & above this pH, enzyme activity is much lower and at extreme pH, enzyme becomes totally inactive.
- Effect of product concentration:
- Product accumulation decreases enzyme velocity.
- In living system, prevention is by quick removal of products formed.
Feedback Inhibition:
- Inhibiting the first step by the final product.
- Effect of activators:
- Inorganic Co-factor necessary to enhance enzyme activity.
- Metallic cations : Mg++,Mn++,Zn++,Ca++,Co++,Cu++
- Anions : Cl- (amylase)
- Effect of time
- Ideal & optimal conditions (pH & temp.) time required is less.
- Effect of light & radiation
- Inactivates certain enzymes due to peroxides formation.
- UV rays inhibit salivary amylase activity.
Enzyme inhibition
Inhibitor I: Substance which binds with E and decreases its catalytic activity.
Enzyme inhibition can be broadly classified into three types:
- Competitive inhibition-
- Non-competitive
- Allosteric inhibition
Competitive Inhibition:
Inhibitor binds non-covalently – Reversible
Competitive Inhibitor:
-
- Substrate analogue
- Binds at active site of enzyme
(Non-covalent) but no catalysis.
E + S → ES ↔ E + P
E + I ↔ EI
Both ES & EI complexes are formed.
-
- As Inhibitor (I) is present at active site, E is not available for S binding.
- I interfere with ES binding: I decrease affinity of E with S: Km value increases.
- Inhibition could be overcome by high S concentration: Vmax unchanged.
Examples of Competitive Inhibition:
Enzyme Succinate dehydrogenase of Kreb’s cycle
Substrate: Succinic acid
Inhibitors: Oxalic acid, Malonic acid, Glutaric acid.
| Inhibitors (Drugs) | Enzyme inhibited | Clinical applications |
| Allopurinol | Xanthine oxidase | Used in treatment of Gout |
| Ethanol | Alcohol dehydrogenase | Used in methanol poisoning |
| Methotrexate | Dihydrofolate reductase | Anti-cancer drug |
| Ephedrine, amphetamine | Monoamine oxidase | Elevates catecholamine levels |
| Sulfonilamide | Dihydropteroate synthase | Antibiotic |
| Dicumarol | Vitamin K epoxide reductase | Anticoagulant |
| Lovastatin | HMG CoA reductase | Use to Lower Cholesterol levels in plasma |
| Succinyl choline | Acetylcholine esterase | Muscle relaxation in surgery |
Antimetabolites:
- Used in Cancer therapy (methotrexate, aminopterin)
- Used in – gout (allopurinol)
Antivitamins: Cause deficiencies
- Dicumarol, Sulfonilamide
Non-Competitive Inhibition
Non-competitive Inhibitor
- No structural resemblance with substrate.
- Binds at other than active site (Covalently): Irreversible inhibition
- I binds E & ES complex EI & ESI complexes formed.
- EI binding doesn’t interfere with ES binding: Km unchanged
- Inhibition cannot overcome by increase in [S] : Vmax decreased.
Clinical Applications- Examples of Non-competitive inhibitors:
Heavy metal ions (Ag+, Pb++, Hg++):
- Inhibit the enzymes ferrochelatase, aminolevulinate dehydratase by binding with cysteinyl sulfhydryl group
- E-SH +(Pb2⁺) → E-S. . .Pb2⁺ +H⁺
Disulfiram : (Antabuse)
- Drug used in the treatment of alcoholism.
- Irreversibly inhibit the enzyme aldehyde dehydrogenase.
- Alcoholics- acetaldehyde accumulation- alcohol avoidance
Organophosphorus insecticides- Malathion
- Inhibit enzyme AchE
- Essential for nerve conduction- paralysis of vital body functions.
Penicillin antibiotics
- Inhibition of serine containing enzymes
- Block bacterial cell wall synthesis.
Iodoacetate
- Irreversible inhibitor of the enzymes like papain and glyceraldehyde 3 phosphate dehydrogenase
Di-isopropyl fluorophosphate (DFP)
- Nerve gas developed by the Germans during second world war.
- DFP irreversibly binds with enzymes containing serine at the active. E.g. serine proteases, acetylcholine esterase.
- Fluoride inhibits Enolase (Glycolysis enzyme).
- Cyanide inhibits cytochrome oxidase (ETC)
Suicide Inhibition:
- “ Mechanism Based” Inhibition
- Specialized form of irreversible inhibition
- Original inhibitor is converted to more potent form by the same enzyme that ought to be inhibited
- Allopurinol à Alloxanthine : Xanthine oxidase
- 5FU à Fluorodeoxyuridylate : thymidylate synthase
- Reversible à irreversible inhibition
Comparison between two types of inhibition:
| Competitive Inhibition | Non-competitive inhibition | |
| Acting on | Active site | Other than active site |
| Structure of Inhibitor | Substrate analogue | Unrelated molecule |
| Inhibition is | Reversible | Generally Irreversible |
| Excess substrate | Inhibition relieved | No effect |
| Km | Increased | No Change |
| Vmax | No change | Decreased |
| Significance | Drug action | Toxicological |
Allosteric Inhibition:
Feedback Inhibition:
- End product inhibition- Specialized type of allosteric inhibition.
- Inhibiting the first step by the final product.
- Feedback inhibitor= Negative allosteric effector
- Cellular economy to save wasteful expenditure.
Iso-Enzyme
The multiple forms of an enzyme catalysing the same reaction are isoenzymes or isozymes.
- All isoenzymes are oligomeric.
Differ in their physical and chemical properties which include
- Structure,
- Electrophoretic and immunological properties,
- Km and V max values,
- pH optimum,
- Relative susceptitibility to inhibitors
- Degree of denaturation.
Isoenzymes of LDH (Lactate Dehydrogenase):
Lactate LDH Pyruvate
Normal levels of LDH in serum are 100-200 U/L.
LDH is an Oligomeric enzyme made up of four polypeptide subunits (tetramer).
Two types of subunits, namely M (for muscle) and H (for heart), are encoded by different genes. They assemble in varying combinations to produce five distinct isoenzymes.
| Isoenzymes | Subunits | Electrophoretic Mobility | Tissue of origin |
| LDH1 | H4 | Fastest (highest negative charge) | Heart |
| LDH2 | H3M | Faster | RBC |
| LDH3 | H2M2 | Fast | Brain |
| LDH4 | HM3 | Slow | Liver |
| LDH5 | M4 | Slowest | Skeletal Muscle |
Clinical significance:
- Normally LDH1: LDH2 ratio- Less than 1.
- In myocardial infarction: Increase in LDH1 activity: Ratio becomes more than 1.
Flipped Ratio: Reversal of ratio from less than 1 in normal individuals to more than 1 in MI.
- Liver diseases with hepatocellular damage like hepatitis: LDH4 & LDH5 rise is more.
- Muscular dystrophies: LDH5 rise is more.
Isoenzymes of CPK or CK (Creatinine Phosphokinase or Kinase):
Phosphocreatinine + ADP CK Creatinine + ATP (Lohmann’s Reaction)
Normal levels of CK: 15-100 U/L for males and 10-80 U/L for females.
CK is a dimer with two subunits B (for brain) and M (for muscle).
| Isoenzymes | Subunits | Electrophoretic Mobility | Tissue of origin |
| CK 1 | BB | Fast moving | Brain |
| CK 2 | MB | Heart | |
| CK 3 | MM | Slow moving | Muscle |
Clinical Significance:
CPK-2:
- Increases in MI upto 20% (normal 2%)
- First enzyme to rise & earliest reliable indicator.
- Magnitude of rise of CPK-MB corresponds approximately to size of muscle infarct indicating severity of MI.
CK (Total):
- Rise in Muscle damage. (crush injuries, muscle dystrophies)
Isoenzymes of Alkaline Phosphatase:
ALP is monomer, iso-enzymes are formed as a result of carbohydrate content variation.
Clinical Significance:
- Normal serum value: 40-125 U/L
- Moderate increase: Hepatic diseases
- Very high levels: Extrahepatic obstruction (Obstructive jaundice)
- Drastically High levels: Bone disorders, Paget’s disease, rickets, osteomalacia, osteoblastoma, metastatic carcinoma of bone
Plasma Enzymes:
Most of the enzymes estimated in plasma are not originally plasma enzymes but are primarily intracellular, and are released into the blood when the cell membrane is damaged. Hence, enzymes measured in plasma can be divided into two groups
1) Plasma specific
2) Plasma non-specific enzymes
- Plasma specific or plasma functional enzymes:
- Enzymes are present & their enzyme activities are higher in plasma
- Mostly synthesized in liver & enter the circulation
- Functional constituents of blood.
- Lipoprotein lipase, plasmin, thrombin, choline esterase, ceruloplasmin
- Plasma non-specific or plasma non-functional enzymes:
- Absent or present at low concentration in plasma.
- Secretary: Digestive enzymes of GI tract present in plasma – amylase, pepsin, trypsin, lipase
- Constitutive: Associated with cell metabolism- LDH, CPK, transaminase, ACP, ALP
- Enzyme estimation in plasma reflects the diagnosis & prognosis of several diseases. (Biomarkers)
Diagnostic Importance of Enzymes:
| Enzyme Increased | Conditions/Disorders |
| ALT/ SGPT | Liver Diseases |
| AST / SGOT | Myocardial Infarction |
| Amylase & Lipase | Acute Pancreatitis |
| Alkaline Phosphatase | Bone disorders, Obstructive jaundice |
| Acid Phosphatase | Carcinoma Prostate |
| Lactate Dehydrogenase | MI, Liver diseases |
| CPK Creatinine Kinase | MI |
| Gamma Glutamyl Transferase GGT | Alcoholism |
| Enzyme Decreased | Conditions/Disorders |
| Amylase | Liver disorders |
| Pseudo cholinesterase
(ChE II) |
Viral hepatitis, malnutrition, liver cirrhosis, liver cancer |
| Ceruloplasmin | Wilson’s disease |
| G6PD in RBC | Congenital deficiency With hemolytic anemia |
Enzyme profile in liver diseases:
-
- Viral hepatitis (jaundice), toxic hepatitis, cirrhosis, necrosis:
Increased: ALT/SGPT (Marked increase), LDH-5, AST/SGOT (Moderate increase)
ALT/SGPT is more liver specific enzyme and is elevated more than AST.
-
- Intrahepatic & extrahepatic cholestasis: Manifested as obstructive jaundice
Increased: Alkaline phosphatase, 5’ –Nucleotidase
-
- Alcoholic liver disease :
Increased: GGT (Gamma glutamyl transpeptidase)
Enzyme Profile in Myocardial Infarction:
- Creatine Kinase (CK-MB): First enzyme to rise after infarction.
- LDH-1: Cardiac specific; But late marker
- SGOT/AST: Not cardiac specific
Cardiac Biomarkers:
| Markers | Characteristics |
| Myoglobin | Earliest cardiac marker, Non specific |
| Cardiac Troponin I & T | Cardiac Specific |
| Creatine Kinase
(CK-MB) |
First Enzymatic Marker, Cardiac Specific |
| Lactate Dehydrogenase (LDH-1) | Enzymatic marker, Cardiac Specific |
| SGOT/AST | Late Enzymatic marker, Non specific |
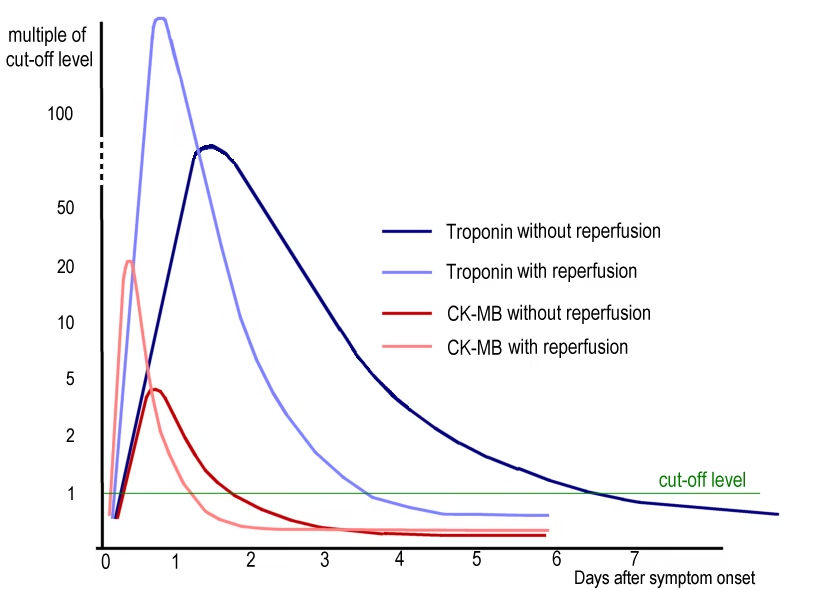 Image: Diagnostic markers in Myocardial infarction
Image: Diagnostic markers in Myocardial infarction
Applications of Enzymes:
Therapeutic Applications:
| Enzymes | Applications |
| Streptokinase/ urokinase | Acute MI, DVT, pulmonary embolism |
| Asparginase | Cancer therapy- leukemia (ALL) |
| Papain | Anti-inflammatory |
| Antitrypsin α-1 | Treatment of emphysema |
Applications in Genetic Engineering:
| Enzymes | Application |
| Restriction Endonuclease | Gene Transfer, DNA fingerprinting, RFLP |
| Taq DNA polymerase | Polymerase chain reaction |
Applications as Analytical Reagents:
| Enzyme | Application in Estimation |
| Urease | Urea |
| Uricase | Uric Acid |
| Cholesterol oxidase | Cholesterol |
| Glucose oxidase-peroxidase | Glucose |
| Lipase | Triglycerides |